Predict the products of the following reactions: (b) CH CHCCHH2CH (a) HCI H (d) CH CH2 (c)
Question:
Predict the products of the following reactions:
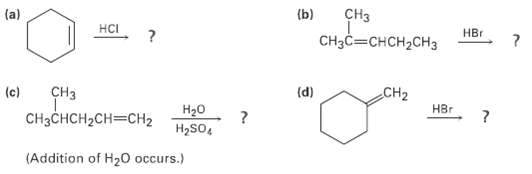
Transcribed Image Text:
(b) CHз CHзCCHсH2CHз (a) HCI Hвг (d) CHз CH2 (c) HBr CнзснсH-CH3CH2 H20 H2SO, (Addition of H20 occurs.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 86% (15 reviews)
Strategy All of these reactions are electrophilic additions of HX to an alkene Use Markov...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the products of the following reactions (the aromatic ring is un-reactive in all cases). Indicate region-chemistry when relevant. H2/Pd (a) Br2 (b) Os04 (c) NMO Cl2, H20 (d) CH212, Zn/Cu (e)...
-
Predict the products of the following reactions: (a) (b) CH CH3CH2CH-0-CH2CH2CH3 CH r HBr
-
Predict the products of the following reactions: CH (b) CH (a) 1. (NH2)2C=S 2. NaOH, H20 -CH CH2CH2CH2Br Hr SCH2CH3 (d) (c) Br2, ? H0z. 2 SH
-
(a) A proton is moving at a speed much slower than the speed of light. It has kinetic energy K1 and momentum P1. If the momentum of the proton is doubled, so P2 = 2p1 how is its new kinetic energy K2...
-
What relevance might contingency leadership have for dealing (a) men versus women, and (b) old versus young?
-
Hoover Electronics has beginning inventory of 22 ,000 units, will sell 60,000 units for the month, and desires to reduce ending inventory to 30 percent of beginning inventory. How many units should...
-
Describe two ratios that relate a firms stock price to its earnings and book value per share and write their equations. AppendixLO1
-
The following equity investment-related transactions were completed by Lance Company in 2010: Jan. 12. Purchased 1,800 shares of Baxter Company for a price of $56.50 per share plus a brokerage...
-
True or false and explain: A mutual fund investing in shares trading on an emerging capital market (say Turkey, for example) creates more value than one investing in shares trading on an established...
-
Discuss and summerize the Hecher Ohlin Model. Specify the assumptions used, definitions made and theorems solved.
-
Name the following alkenes, and tell which compound in each pair is more stable CH {a) H2C=CHCH2CH3 or H CH2CH2CH3 (b) C=C or H3C CH-H,CH C CH (c) or
-
What alkenes would you start with to prepare the following alkylhalides? CH-CH (a) (b) Br Br CI (c) (d) CH3CH2CHCH2CH2CH3
-
A heavily insulated cylinder/piston contains ammonia at 1200 kPa, 60C. The piston is moved, expanding the ammonia in a reversible process until the temperature is 20C. During the process 600 kJ of...
-
What are electromagnetic moments?
-
How do we design a superconducting synchronous motor?
-
How do we design a superconducting induction motor?
-
How do we design a superconducting cyclo converter?
-
How do we design a superconducting non-inverting op amp circuit?
-
Do you believe Carolyn is eligible for unemployment compensation?
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
Methylamine has a vapor pressure of 344 torr at -25 C and a boiling point of -6.4 C. Find Hvap for methylamine.
-
In each of the following processes, give the products and classify each of the groups indicated by a colored label with one or more of the following terms: Bronsted base, Lewis base, Bronsted acid,...
-
The examples of incorrect curved-arrow notation in Fig. P3.36 were found in the notebooks of Barney Bottle brusher, a student who was known to have difficulty with organic chemistry. Explain what is...
-
Use the curved - arrow notation to derive three other resonance structures for anthracene. CC1..thmatummail three additional structures anthracene
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


