Predict the products of the following reactions (the aromatic ring is un-reactive in all cases). Indicate region-chemistry
Question:
Predict the products of the following reactions (the aromatic ring is un-reactive in all cases). Indicate region-chemistry when relevant.
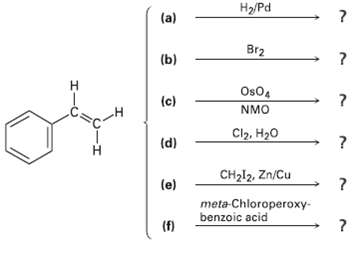
Transcribed Image Text:
H2/Pd (a) Br2 (b) Os04 (c) NMO Cl2, H20 (d) CH212, Zn/Cu (e) meta-Chloroperoxy- benzoic acid (f)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
a b O c I CCH CCH HIC CCH HPd B...View the full answer

Answered By

Rehab Rahim
I am well versed in communicating and teaching in areas of all business subjects. I have helped many students in different ways from answering answers to writing their academic papers.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the products of the following reactions: (a) (b) CH CH3CH2CH-0-CH2CH2CH3 CH r HBr
-
Predict the products of the following reactions: CH (b) CH (a) 1. (NH2)2C=S 2. NaOH, H20 -CH CH2CH2CH2Br Hr SCH2CH3 (d) (c) Br2, ? H0z. 2 SH
-
Predict the products of the following ozonolysis reactions. (a) (b) (c) (1) O2 (2) Me2S (1) 03 (2) Me S (1) O3 (2) Me S
-
Smart housing Inc. is negotiating a deal to build a house. The owner wants to start in early spring when the weather begins to moderate and build through the summer into the fall. The completion time...
-
High school students across the nation compete in a financial capability challenge each year by taking a National Financial Capability Challenge Exam. Students who score in the top 20 percent are...
-
Compute (a) ||A|| 2 and (b) cond 2 (A) for the indicated matrix. A in Exercise 3 Data From Exercise 3 A =
-
17. Assume that your friend has accepted a position working as an accountant for a large automaker. As a signing bonus, the employer provides the traditional cash incentive but also provides the...
-
Harris Company, which began operations in 2013, invests its idle cash in trading securities. The following transactions relate to its short- term investments in its trading securities. 2013 Mar. 10...
-
Suppose the bond in previous question 3 (where Michael bought a bond with $1,000 par value paying a 5% annual coupon) is called two years later with the call premium of 9%. Assuming its yield to...
-
Locate Revenue Ruling 2018-20. Explain the effect of that ruling on previous Treasury Department pronouncements.
-
From what alkene was the following 1, 2-diol made, and what method was used, epoxide hydrolysis or OsO4?
-
Suggest structures for alkenes that give the following reaction products. There may be more than one answer for some cases. H (b) CH CH3 /Pd CH2H2H2CH H2/Pd (d) CH Br CHH2CHCH (c) Br2 HCI ,...
-
What is a firms target cost? How is it used during the new product development stage?
-
Dr. Kovaleski is interested in examining whether quantity of sleep impacts problem solving ability. To test problem solving ability, the research team gave participants a puzzle and measured how long...
-
Can you please help me fill out the spreadsheet? Idexo Corporation is a privately held designer and manufacturer of licensed college apparel in Cincinnati, Ohio. In late 2020, after several years of...
-
CHECK FIGURE: Adjusted book balance = $2,837.06 Mae Telford, the controller of the Baylor Company, provided the following information: Bank statement balance Add: Baylor Company Bank Reconciliation...
-
Read the Scenario Congratulations, you are now the Police Chief in Anytown, USA. A city with 30,000 residents and you are responsible to provide 24 hour a day police coverage. You have a total of 45...
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x = 3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
What are the accounting bases of controlling manufacturing overhead costs, and what is the position of each within the framework of cost control? LO.1
-
In the busy port of Chennai, India, the number of containers loaded onto ships during a 15-week period is as follows: 1. Develop a linear trend equation to forecast container loadings. 2. Using the...
-
One of these substances is a liquid at room temperature. Which one? a) CH 3 OH b) CF 4 c) SiH 4 d) CO 2
-
Ethyl bromide (0.IM) and HBr (0.1 M) are allowed to react in aqueous THE with 1 M sodium cyanide (Na+ -CN). What products are observed? Arc any products formed more rapidly than others? Explain.
-
What is the expected substitution product (including its stereochemical configuration) in the SN2 reaction of potassium iodide in acetone solvent with the following compound? (D = 2H = deuterium, an...
-
(a) Give the structure of the S*2 reaction product between ethyl iodide and potassium acetate. H,C-C potassium acetate
-
Describe how the following affect the valuation of PPE. a) Cash Discounts b) Deferred Payment Contracts
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. His annual pay raises are determined by his division s...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.

Study smarter with the SolutionInn App


