For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic,
Question:
(a)
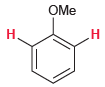
(b)
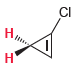
(c)
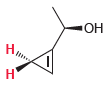
(d)
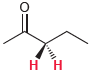
(e)
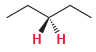
Discuss.
Transcribed Image Text:
OMe Н. Н CI H,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
a Homotopic b ...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following compounds, draw the important resonance forms. Indicate which structures are major and minor contributors or whether they have the same energy. (a) H2CNN (b) H2C=CH-NO2 (c)...
-
For each of the following compounds, give the systematic name and the common name (for those that have common names), and indicate whether the amines are primary, secondary, or tertiary: a. b. c....
-
For each of the following compounds, use the nitrogen rule to determine whether the molecular weight should be even or odd. Then calculate the expected m/z value for the molecular ion. a. b. c. d. O:...
-
Engineering is a dynamic field that requires continuous learning. Discuss how you plan to acquire and apply new knowledge as needed throughout your engineering career. Address the strategies you...
-
What is intellectual property? What are three examples of intellectual properties?
-
Simulate the mini-mart situation described in Problem 45 of Chapter 11. Identify the best order quantity using a simulation with 100 trials. Data from Problem 45 of Chapter 11 A gasoline mini-mart...
-
Describe the discipline of organizational change management. AppendixLO1
-
You are considering opening a copy service in the student union. You estimate your fixed cost at $15,000 and the variable cost of each copy sold at $.01. You expect the selling price to average $.05....
-
The market consensus is that Analog Electronic Corporation has an ROE = 9%, a beta of 1.65, and plans to maintain indefinitely its traditional plowback ratio of 2/3. This year's earnings were $2.20...
-
As the accountant for Veneskey & Sons, you have been hired to prepare the payroll and everything that goes along with it for OlFashion Industries which has 4 employees. Their necessary payroll...
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
Describe briefly three situations in which debit entries may properly be made in accumulated depreciation accounts. LO2
-
If a change were made to Technical Spec 2 in the product's design, this would likely change the customer's opinion of which value feature the most? Quick Start Quick Start QFD Matrix 1 = Strong...
-
You are a quality management consultant for the Beserk Tennis Ball Company. Beserk is redesigning its current model of tennis ball, and you are asked to use QFD analysis to make suggestions about...
-
You are reviewing a tender evaluation that is to be awarded on lowest total price. The bid evaluations follow: To which company should the contract be awarded? Company Capital Cost Maintenance...
-
You have invited four companies to bid on a consulting project. All four companies answered your invitation to tender, but the bids vary in the number of hours each company estimates will be required...
-
Boston Cycles inventory data for the year ended December 31, 2011, follow: Assume that the ending inventory was accidentally overstated by $2,200. Requirement 1. What are the correct amounts for cost...
-
Describe verbally the inverse of the statement. Then express both the given statement and its inverse symbolically. Take the cube root of x and add 1.
-
1) The government decided to reduce taxes on fast-food to increase revenue. The government assumes that fast-food products have a) An inelastic demand b) An elastic demand c) A demand curve that is...
-
Normally, a Trans alkene is more stable than its cis isomer Trans-Cyclooctene, however, is less stable than cis-Cyclooctene by 38.5kJ/mol. Explain.
-
Trans-Cyclooctene is less stable than cis-Cyclooctene by 38.5kJ/mol, but Trans cyclononene is less stable than cis-cyclononene by only 12.2kJ/mol. Explain.
-
Allene (1, 2-propadiene), H2C = C = CH2, has two adjacent double bonds. What kind of hybridization must the central carbon have? Sketch the bonding orbitals in allene. What shape do you predict for...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


