In our discussion of bonding, we have not encountered a bond order higher than triple. Use the
Question:
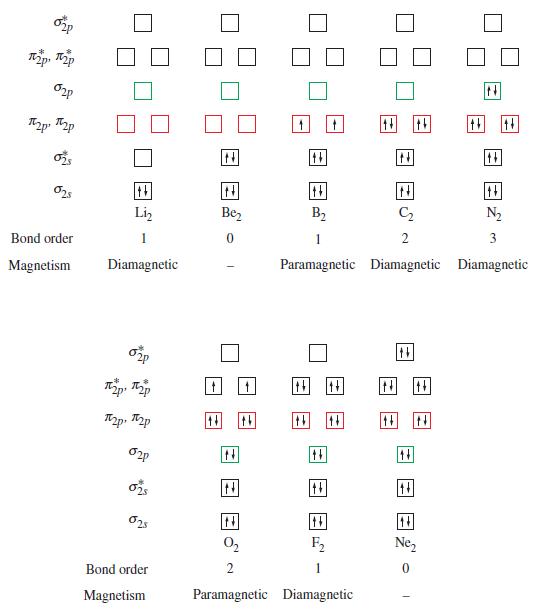
In our discussion of bonding, we have not encountered a bond order higher than triple. Use the energy level diagrams of Figure 11-26 to show why this is to be expected.
Figure 11-26
Transcribed Image Text:
820 кар, кар 020 2p p 025 025 Bond order Magnetism ++ Li₂ 1 Diamagnetic 020 p p p. p 020 025 025 Bond order Magnetism N |tt| Be₂ 0 N th 44 ++ |N| B₂ C₂ 1 2 Paramagnetic Diamagnetic +4 ++ N 0₂ 2 Paramagnetic Diamagnetic ++ F₂ 14 N 14 O 14 N₂ 3 Diamagnetic
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In our discussion of the reaction e + + e μ + + μ , we completely neglected the Higgs exchange diagram of Figure 10.30, compared with the dominant diagrams of Figures...
-
In our discussion of economic versus statutory incidence, the text has focused primarily on the incidence of taxes. This exercise explores analogous issues related to the incidence of benefits from...
-
In our discussion of Figure 12.4, we assumed that the monopoly engaged in block-pricing by setting both block prices so that they were on the demand curve. However, suppose the monopoly sets the...
-
College Spirit sells sportswear with logos of major universities. At the end of 2019, the following balance sheet account balances were available. Accounts payable $104,700 Required: 1. Prepare a...
-
Caleb, operator of a window-washing business, dictated a letter to his secretary addressed to Apartments, Inc., stating, I will wash the windows of your apartment buildings at $4.10 per window to be...
-
What is the purpose of modelling in business administration research?
-
LO5 Sidney lives in Hayes, Kansas. He owns land in Cotulla, Texas, that he inherited from his father several years ago. The land is unimproved and has never produced income. On January 26, 2010,...
-
During the first month of operations ended August 31, Kodiak Fridgeration Company manufactured 80,000 mini refrigerators, of which 72,000 were sold. Operating data for the month are summarized as...
-
13- On January 4, XYZ Company purchased a machine for $1,700 and supplies (to be used in the following 2 years) for $400 from Milkman Company. Milkman agreed to receive his balance after 30 days. *...
-
Coated metallic disks are cured by placing them at the top of a cylindrical furnace whose bottom surface is electrically heated and whose sidewall may be approximated as a reradiating surface. Curing...
-
Is it correct to say that when a diatomic molecule loses an electron, the bond energy always decreases (that is, that the bond is always weakened)? Explain.
-
N 2 (g) has an exceptionally high bond energy. Would you expect either N 2 - or N 2 2- to be a stable diatomic species in the gaseous state? Explain.
-
We discuss the growing diversity of the workforce. However, if you grew up in a fairly homogeneous town and went to a fairly homogeneous school, you may not have had much experience with diversity....
-
The expected annual net income is $200,000; the average investment is $800,000; and depreciation expense is $50,000. Calculate the annual rate of return.
-
How do you define humanities?
-
Write the types of partners ?
-
What are electromagnetic moments?
-
How do we design a superconducting synchronous motor?
-
Write the structural formula of the product expected from the reaction of CH3CC-Na+ with 3-pentanone followed by H3O+.
-
When the concentration of a strong acid is not substantially higher than 1.0 10-7 M, the ionization of water must be taken into account in the calculation of the solution's pH. (a) Derive an...
-
Dividends and Taxes Sharp Dress, Inc has declared a $5.00 per share dividend, suppose capital gains are not taxed, but dividends are taxed at 15 percent. New IRS regulations require that taxes be...
-
Stock Dividends the owner's equity accounts for Quadrangle International are shown here: a. If Quadrangle stock currently sells for $25 per share and a 10 percent stock dividend is declared, how many...
-
Stock Splits for the company in problem 2, show how the equity accounts will change if. a. Quadrangle declares a four-for-one stock split. How many shares are outstanding now? What is the new par...
-
SkyChefs, Incorporated, prepares in-flight meals for a number of major airlines. One of the company's products is grilled salmon with new potatoes and mixed vegetables. During the most recent week,...
-
Tentacle Television Antenna Company provided the following manufacturing costs for the month of June. Direct labor cost $132,000 Direct materials cost 84,000 Equipment depreciation (straight-line)...
-
At the beginning of the year, Vendors, Inc., had owners' equity of $48,875. During the year, net income was $5,275 and the company paid dividends of $3,775. The company also repurchased $7,625 in...

Study smarter with the SolutionInn App


