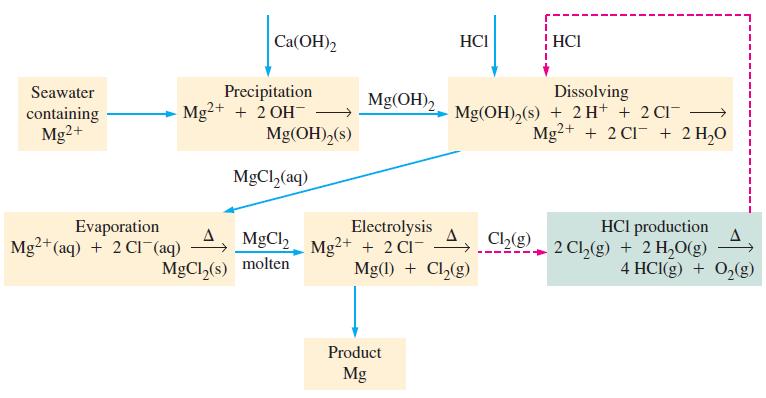
In the Dow process (Fig. 21-13), the starting material is Mg 2+ in seawater and the final
Question:
In the Dow process (Fig. 21-13), the starting material is Mg2+ in seawater and the final product is Mg metal. This process seems to violate the principle of conservation of charge. Does it? Explain.
Figure 21-13
Transcribed Image Text:
Seawater containing Mg2+ Ca(OH)2 Precipitation Mg²+ + 2 OH- Evaporation Mg2+ (aq) + 2 CI (aq) Mg(OH)2 (s) MgCl₂(aq) MgCl, MgCl₂(s) molten Mg(OH)₂ Electrolysis Mg²+ + 2 CI- HCI Product Mg Mg(1) + Cl₂(g) HCI Dissolving Mg(OH)₂(s) + 2 H+ + 2 CI¯ Mg2+ + 2 C1 + 2 H₂O HCI production Chg 2 Ch₂(g) + 2 H₂O(g) Ch₂(g) 1 4 HCl(g) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
No the Dow process does not violate the principle of conservation of charge The process starts with ...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Q1. How worried are clients and stakeholders in day-to- day product improvement? 2. the industrial corporation Case for Agility "The struggle is not always to the most powerful, nor the race to the...
-
When o-chlorotoluene is subjected to the conditions used in the Dow process (i.e., aqueous NaOH at 350oC at high pressure), the products of the reaction are o-cresol and m-cresol. What does this...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
2. National Defense (40 points). There are 11 countries in Europe who get utility from general consumption c, and from European national defense G. The utility of a generic country i is u(ci, G) =...
-
Refer to PE 410. 1. Make the adjusting entry necessary on the companys books on December 31 with respect to this insurance policy 2. Compute the ending balance in the prepaid insurance account....
-
The unadjusted trial balance and adjustment data for Elbow Cycle Repair Shop are presented in P4-2A. Instructions Prepare a work sheet for the year ended January 31, 2014. Taking It Further Is it...
-
Identify the five steps involved in segmenting and targeting markets.
-
Uncle Butchs Hunting Supply Shop reports the following information related to inventory: Calculate Uncle Butchs Hunting Supply Shops ending inventory using the retail inventory method under the FIFO...
-
Question 8 Which of the following items should be included in gross income? 1) Life insurance proceeds 2) Child support payments 3) Accident and health insurance proceeds 4) Cash rebate from a dealer...
-
Without performing detailed calculations, indicate why you would expect each of the following reactions to occur to a significant extent as written. Use data from Appendix D as necessary. (a)...
-
To prevent the air oxidation of aqueous solutions of Sn 2+ to Sn 4+ , metallic tin is sometimes kept in contact with the Sn 2+ (aq). Suggest how this contact helps prevent the oxidation.
-
True or False Every equation of the form x 2 + y 2 + ax + by + c = 0 has a circle as its graph.
-
What is the average age (measured by the variable "age") of the sample in the GSS93 subset.sav data set? Is there a significant difference in the age of those who favor the death penalty for murder...
-
Solve the system of linear equations, using the Gauss-Jordan elimination method. (If there is no solution, enter NO SOLUTION. If there are infinitely many solutions, express your answer in terms of...
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Prepare Balance Sheet: To do this activity you are required to assume the amount and line items that are to be shown on the balance sheet of your business selling homemade articles. Using the...
-
You have a "Consent to Use E-mail Communication" on file for this patient. Draft a short e-mail to her about her lab and chest X-ray results, requesting she contact the office by phone or e-mail to...
-
Apply the Edmonds-Karp and Ford-Fulkerson algorithms to find a maximum flow in Examples 13.12, 13.13, and 13.14.
-
Study the pictures/images below. Obviously these was focus on LT sociology, anthropology and poltical science. Try to do some analysis by finding clues that are synonymous with the main concepts....
-
Kopke Company, organized in 2012, has these transactions related to intangible assets in that year: Jan. 2 Purchased a patent (5-year life) $280,000. Apr. 1 Goodwill acquired as a result of purchased...
-
Alliance Atlantis Communications Inc. changed its accounting policy to amortize broadcast rights over the contracted exhibition period, which is based on the estimated useful life of the program....
-
The questions listed below are independent of one another. Instructions Provide a brief answer to each question. (a) Why should a company depreciate its buildings? (b) How can a company have a...
-
Discuss American History
-
Your firm has developed a new lithium ion battery polymer that could enhance the performance of lithion ion batteries. These batteries have applications in many markets including cellphones, laptops,...
-
Need help analyzing statistical data 1. ANOVA) True or false: If we assume a 95% confidence level, there is a significant difference in performance generally across all groups. 2. (t-test) True or...

Study smarter with the SolutionInn App


