In the following diagrams, which representation demonstrates a weak electrolyte? Gas Aqueous Gas Aqueous Gas Aqueous Gas
Question:
In the following diagrams, which representation demonstrates a weak electrolyte?
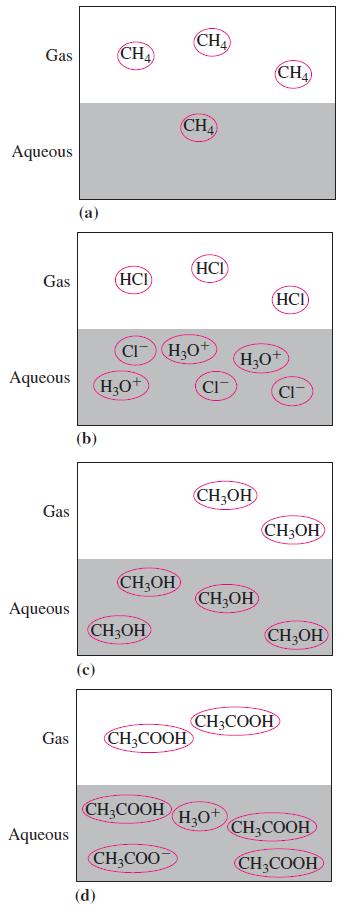
Transcribed Image Text:
Gas Aqueous Gas Aqueous Gas Aqueous Gas Aqueous (a) (b) CH4 (HCI (d) H₂O CH3OH CH₂OH CI-H₂O+ CH₂COOH CH3COOH CH₂COO CHA CHA (HCI) CI CH₂OH CH₂OH H₂O+ H3O+ CHA (HCI) CH3COOH CIT CH3OH CH₂OH CH₂COOH CH₂COOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The diagram that demonstrates a weak electrolyte is d Weak electrolytes are substances that partiall...View the full answer

Answered By

Isaiah Mutinda
As a graduate with Bs in Maths and Computer Science and having worked as a freelance full stack software developer for 3 years running I believe I have what it takes to conformable tutor and mentor a student to a professional developer also.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
In the following diagrams, which representation demonstrates a strong electrolyte? Gas Aqueous (a) Gas Aqueous Gas Aqueous (b) Aqueous (c) CHA (HCI) HO+ CHOH (d) CI) H0+ CHOH CHA Gas CH3COOH CHA...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
In Problems 25 54, solve each system. Use any method you wish. 2xxy + y = 8 xy = 4
-
Wallowa Company is considering a long-term investment project called ZIP. ZIP will require an investment of $120,000. It will have a useful life of 4 years and no salvage value . Annual cash inflows...
-
Assuming they began by opening just one or two stores in Mexico, what do you see as the main HR-related challenges Jack and Jennifer would have to address?
-
Why is a call provision advantageous to a bond issuer? When would the issuer be likely to initiate a refunding call? AppendixLO1
-
At year-end 2010, the trial balance of Pennopscott Corp. showed the following accounts and amounts: Assume that, taken together, the variances are believed to be significant. Prepare the journal...
-
Connor Company's budgeted price for direct materiale, direct manufacturing labor, and deact marketing (debiton) laber per atache cer* 144,17.11.es The president is pleased with the following...
-
An isotonic solution is described as 0.92% NaCl (mass/volume). Would this also be the required concentration for isotonic solutions of other salts, such as KCl, MgCl 2 , or MgSO 4 ? Explain.
-
NH 3 (aq)conducts electric current only weakly. The same is true for acetic acid, CH 3 COOH. When these solutions are mixed, however, the resulting solution conducts electric current very well....
-
An investment has an outlay of 100 and after-tax cash flows of 40 annually for four years. A project enhancement increases the outlay by 15 and the annual after-tax cash flows by 5. As a result, the...
-
Two years ago, Dave started a newly incorporated business with a strict policy of paying half the current annual profits to shareholders as dividends on Dec 3 1 % . if he generated a loss of 5 2 , 2...
-
Nancy Inc. has the following data to prepare budgets for 2023: Budgeted Sales units: 1st Qtr 8,000; 2nd Qtr 8,500; 3rd Qtr 9,000, 4th Qtr 9,500 Selling price per unit is $35 Desired Ending Finished...
-
Given a binary tree shown below, please identify: (15 points) (a) All the leaf nodes; (b) All the internal nodes; (c) Siblings of node 5; (d) Height of the tree; (e) Length of path of node 3 to node...
-
CONFIDENTIAL QUESTION 3 AC/JUN 2019/ACC406 Shaza is the owner of Shazayra Enterprise, a sport boutique. The business is engaged in selling various sport items and located at a strategic location. She...
-
An investor owns 30% of the equity of an associate. During the year, the associate sells inventory to the investor that has a cost of $103,680. A mark up at cost of 25% was added to arrive at the...
-
Find the Laplace transform of the following functions: (a) t cos t u (t) (b) e-t t sin t u (t) (c) sin t/t u(t)
-
Show that gj concave AHUCQ Abadie For nonnegative variables, we have the following corollary.
-
Draw up an outline or flowchart tracing the capital budgeting process from the initial idea for a new investment project to the completion of the project and the start of operations. Assume the idea...
-
Compare typical compensation and incentive arrangements for (a) Top management, for example, the CEO or CFO, and (b) Plant or division managers. What are the chief differences? Can you explain them?
-
Suppose all plant and division managers were paid only a fixed salaryno other incentives or bonuses. a. Describe the agency problems that would appear in capital investment decisions. b. How would...
-
ABC Corp currently has a debt to enterprise value ratio of 51%. The firm's cost of equity is 8.4% and its cost of debt is 4%. Assuming perfect markets, calculate the unlevered cost of capital for ABC...
-
Rebound Airlines was hurt in the recession, with its stock falling to $10 from $50. The stock has started to recover and has just crossed its 50-day moving average at $20. The 200-day moving average...
-
Biotech Corp has no sales or earnings but is working on a COVID cure. They also have some good prospects for other drugs that could potentially be blockbusters. The stock started the year at $30 and...

Study smarter with the SolutionInn App


