Question: In this problem, we describe an alternative method for balancing equations for oxidation-reduction reactions. The method is similar to the method given previously in Tables
In this problem, we describe an alternative method for balancing equations for oxidation-reduction reactions. The method is similar to the method given previously in Tables 5.5 and 5.6, but it places more emphasis on the assignment of oxidation states. (The method summarized in Tables 5.5 and 5.6 does not require you to assign oxidation states.) An emphasis on oxidation states is warranted because oxidation states are useful not only for keeping track of electrons but also for predicting chemical properties. The method is summarized in the table above. The method offers a couple of advantages. First, the method applies to both acidic and basic environments because we balance charges by using either H+ (for acidic environments) or OH- (for basic environments). Second, the method is somewhat more efficient than the method we described previously because, in the method described here, we balance only once for charge and only once for hydrogen and oxygen. In the other method, we focus on the half-equations separately and must balance twice for charge and twice for hydrogen and oxygen. Use the alternative method described above to balance the following oxidation-reduction equations.
(a) Cr2O72-(aq) + Cl-(aq) → Cr3+(aq) + Cl2(g) (acidic solution)
(b) C2O42-(aq) + MnO4-(aq) → CO32-(aq) + MnO2(s) (basic solution)
Tables 5.5
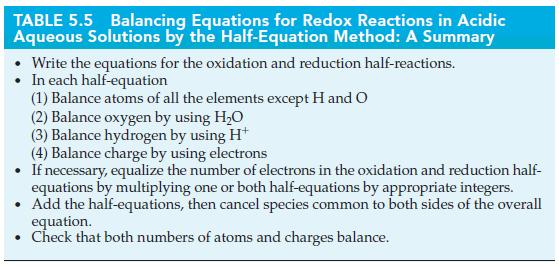
Tables 5.6
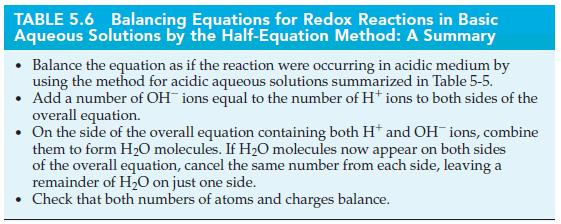
TABLE 5.5 Balancing Equations for Redox Reactions in Acidic Aqueous Solutions by the Half-Equation Method: A Summary Write the equations for the oxidation and reduction half-reactions. In each half-equation (1) Balance atoms of all the elements except H and O (2) Balance oxygen by using HO (3) Balance hydrogen by using H (4) Balance charge by using electrons If necessary, equalize the number of electrons in the oxidation and reduction half- equations by multiplying one or both half-equations by appropriate integers. Add the half-equations, then cancel species common to both sides of the overall equation. . Check that both numbers of atoms and charges balance.
Step by Step Solution
3.54 Rating (154 Votes )
There are 3 Steps involved in it
To balance the equation Cr2O72aq Claq Cr3aq Cl2g in acidic solution using the alternative method Assign oxidation states to all atoms Cr2O72 CrVI OII ... View full answer

Get step-by-step solutions from verified subject matter experts


