One method of obtaining chromium metal from chromite ore is as follows. After reaction (23.16), sodium chromate
Question:
One method of obtaining chromium metal from chromite ore is as follows. After reaction (23.16), sodium chromate is reduced to chromium(III) oxide by carbon. Then the chromium(III) oxide is reduced to chromium metal by silicon. Write plausible equations to describe these two reactions.
Reaction (23.16)
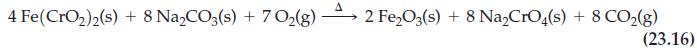
Transcribed Image Text:
4 Fe(CrO₂)2(s) + 8 Na₂CO3(s) + 70₂(g) 2 Fe₂O3(s) + 8 Na₂CrO4(s) + 8 CO₂(g) (23.16)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Reduction of sodium chromate to chromiumIII oxide by carbon 2 Na2CrO4s 3 Cs Cr2O3s 4 N...View the full answer

Answered By

Jehal Shah
I believe everyone should try to be strong at logic and have good reading habit. Because If you possess these two skills, no matter what difficult situation is, you will definitely find a perfect solution out of it. While logical ability gives you to understand complex problems and concepts quite easily, reading habit gives you an open mind and holistic approach to see much bigger picture.
So guys, I always try to explain any concept keeping these two points in my mind. So that you will never forget any more importantly get bored.
Last but not the least, I am finance enthusiast. Big fan of Warren buffet for long term focus investing approach. On the same side derivatives is the segment I possess expertise.
If you have any finacne related doubt, do reach me out.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
One method of obtaining an estimate of the term structure of interest rates is called bootstrapping. Suppose you have a one-year zero coupon bond with a rate of r1 and a two-year bond with an annual...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
A spring is at rest in the vertical direction. When a 5 kg mass is placed upon the spring, the length of the spring compresses to 0 . 2 0 meters. The 5 kg mass is removed and replaced by an 8 kg...
-
The following information was taken from the records of Dallen Company for the year 2013: Sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
Instructions From SEDAR (www.sedar.com) or the company websites, access the financial statements of Loblaw Companies Limited for its year ended January 3, 2015, and Empire Company Limited for its...
-
Describe project quality management (PQM) in terms of planning for quality, quality assurance, and quality control to continuously improve the projects products and supporting processes. AppendixLO1
-
Aric Toll owns and manages the Balboa Island Village Inn, a restaurant and bar in Newport Beach, California. Anne Lemen owns the Island Cottage, a residence across an alley from the Inn. Lemen often...
-
the company Honingway production co. applies factory overhead to production basis of direct labor costs. Assume that at the beginning of the current year estimated that direct material costs would be...
-
Calcium will reduce MgO(s) to Mg(s) at all temperatures from 0 to 2000 C. Use this fact, together with the melting point (839 C) and boiling point (1484 C) of calcium, to sketch a plausible graph of ...
-
You are given these three reducing agents: Zn(s), Sn 2+ (aq), and I (aq). Use data from Appendix D to determine which of them can, under standard-state conditions in acidic solution, reduce (a) Cr 2...
-
A company estimates that the marginal revenue (in dollars per unit) realized by selling x units of a product is 48 - 0.0012x. Assuming the estimate is accurate, find the increase in revenue if sales...
-
The curved rod has a diameter \(d\). Determine the vertical displacement of end \(B\) of the rod. The rod is made of material having a modulus of elasticity of \(E\). Consider only bending strain...
-
If the inertial measurement system were written in C++ according to the design fragment described in Chapter 5, describe the testing strategy you would use. If possible, try to design some test cases.
-
Determine the displacement at point \(C\) of the W14 \(\times 26\) beam made from A992 steel. 8 kip A -5 ft 5 ft. B C -5 ft 5 ft- 8 kip D
-
The beam is subjected to the loading shown. Determine the slope at \(B\) and displacement at \(C\). \(E I\) is constant. Ta Mo C b B
-
A mass, connected to a damper as shown in Fig. 14.30, is subjected to a force \(F(t)\). Find the frequency-response function \(H(\omega)\) for the velocity of the mass. m F(t) y(1) FIGURE 14.30...
-
Find the order of each element in the group of rigid motions of (a) The equilateral triangle; and (b) The square.
-
A handrail, which weighs 120 N and is 1.8 m long. was mounted to a wall adjacent to a small set of steps (Figure P4.26). The support at A has broken, and the rail has fallen about the loose bolt at 8...
-
During the month of March, Lavonis Companys employees earned wages of $64,000. Withholdings related to these wages were $4,896 for Social Security (FICA), $7,500 for federal income tax, $3,100 for...
-
Season tickets for the Panthers are priced at $320 and include 16 games. Revenue is recognized after each game is played. When the season began, the amount credited to Unearned Ticket Revenue was...
-
Sprague Company Ltd. publishes a monthly sports magazine, Fishing Preview. Subscriptions to the magazine cost $28 per year. During November 2012, Sprague sells 6,300 subscriptions for cash, beginning...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


