Only a tiny fraction of the diffusible ions move across a cell membrane in establishing a Nernst
Question:
Only a tiny fraction of the diffusible ions move across a cell membrane in establishing a Nernst potential, so there is no detectable concentration change. Consider a typical cell with a volume of 10-8 cm3, a surface area (A) of 10-6 cm2, and a membrane thickness (l) of 10-6 cm. Suppose that [K+] = 155 mM inside the cell and [K+] = 4 mM outside the cell and that the observed Nernst potential across the cell wall is 0.085 V. The membrane acts as a charge-storing device called a capacitor, with a capacitance, C, given by
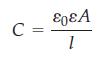
where ε0 is the dielectric constant of a vacuum and the product ε0ε is the dielectric constant of the membrane, having a typical value of 3 x 8.854 x 10-12 C2 N-1 m-2 for a biological membrane. The SI unit of capacitance is the farad, 1 F = 1 coulomb per volt = 1 C V-1 = 1 x C2 N-1 m-1.
(a) Determine the capacitance of the membrane for the typical cell described.
(b) What is the net charge required to maintain the observed membrane potential?
(c) How many K+ ions must flow through the cell membrane to produce the membrane potential?
(d) How many K+ ions are in the typical cell?
(e) Show that the fraction of the intracellular K+ ions transferred through the cell membrane to produce the membrane potential is so small that it does not change [K+] within the cell.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





