Propane (C 3 H 8 ) gas (d = 1.83 kg/m 3 ) is used in most
Question:
Propane (C3H8) gas (d = 1.83 kg/m3) is used in most gas grills. What volume (in liters) of propane is needed to generate 273.8 kJ of heat?
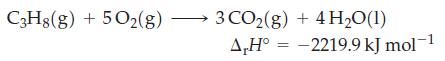
Transcribed Image Text:
C3H8(g) + 5O2(g) 3 CO₂(g) + 4H₂O(1) A,H° -2219.9 kJ mol-1 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
To find the volume of propane C3H8 needed to generate 2738 kJ of heat you can use the following step...View the full answer

Answered By

Geoffrey Isaboke
I am an industrious tutor with a 5-yr experience in professional academic writing. I have passion for History and Music and I have good knowledge in Economics
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Hydrogen gas is a valuable product, because it is used as a feedstock (starting material) for many chemical processes. A common way to produce high-purity hydrogen gas is by reaction of propane gas...
-
The American Society for Quality (ASQ) conducted a salary survey of all its members. ASQ members work in all areas of manufacturing and service-related institutions, with a common theme of an...
-
Identify the usual sequence of manufacturing activities by filling in the blank (with 1, 2, or 3) corresponding to its order: _______ Production activities; _______ sales activities; _______...
-
The following sentences interpret the table in Figure 25. Analyze each sentence to determine whether it represents the data in the table accurately. a. Males and females alike believe Apex is an...
-
WACC AND PERCENTAGE OF DEBT FINANCING Hook Industries capital structure consists solely of debt and common equity. It can issue debt at rd 11%, and its common stock currently pays a $2 00 dividend...
-
You are the new manager of the local GreatBuy Electronics store. Top management of GreatBuy Electronics is convinced that management training should include the active participation of store managers...
-
Ronald Weasley Enterprises sold 4,500 gnomes during the month of August at a price of $7/gnome. Ron offers a full refund to unsatisfied customers for any gnome returned within 30 days from the date...
-
You are to create a direct messenger program. In particular, the program will perform as follows: 1. It must behave as either a client or a server, depending on the command line arguments supplied...
-
What mass of ice can be melted with the same quantity 3.50 mol H 2 O(l) of heat as required to raise the temperature of by 50.0 C? [ fus H = 6.01 kJ/mol H 2 O(s)].
-
The heat of neutralization of HCl(aq) by NaOH(aq) is -55.84 kJ/mol H 2 O produced. If 50.00 mL of 1.05 M NaOH is added to 25.00 mL of 1.86 M HCl, with both solutions originally at 24.72 C, what will...
-
The assembly department has the following production data for the current month. Materials are entered at the beginning of the process. The ending work in process units are 70% complete as to...
-
The figure shows two parts of the graph of a continuous differentiable function f on [10, 4]. The derivative f is also continuous. To print an enlarged copy of the graph, go to MathGraphs.com. (a)...
-
Does your list of 10 products include items with components that are both domestically made and foreign made? Demography is the study of the structure of human populationstheir size, age composition,...
-
Verify the Divergence Theorem for the vector field and region. \(\mathbf{F}(x, y, z)=\langle z, x, yangle\), the box \([0,4] \times[0,2] \times[0,3]\) THEOREM 1 Divergence Theorem Let S be a closed...
-
Let \(\mathbf{F}=\langle 0,-z, 1angle\). Let \(\mathcal{S}\) be the spherical cap \(x^{2}+y^{2}+z^{2} \leq 1\), where \(z \geq \frac{1}{2}\). Evaluate \(\iint_{\mathcal{S}} \mathbf{F} \cdot d...
-
Describe the four different types of competition in the private enterprise system. In which type of competition would each of the following businesses be likely to engage? a. United Airlines b. small...
-
Discuss some of the techniques used to cut fiber-reinforced composites.
-
For the following exercises, write the polynomial function that models the given situation. Consider the same rectangle of the preceding problem. Squares of 2x by 2x units are cut out of each corner....
-
Long-Term Contract with an Overall Loss On July 1, 2010, Torvill Construction Company Inc. contracted to build an office building for Gumbel Corp. for a total contract price of $1,900,000. On July 1,...
-
Installment-Sales Computations and Entries Presented below is summarized information for Johnston Co., which sells merchandise on the installment basis. (a) Compute the realized gross profit for each...
-
Installment-Sales Income Statements Chantal Stores sells merchandise on open account as well as on installment terms. Below are the data pertaining to the construction period? From the data above,...
-
The holder of a share of 12 percent, $100 par-value preferred stock would receive a dividend of __________ per share before any dividend was paid to common stockholders
-
According to IFRS, the pension obligation should be based on Select one: a. the remaining years of serviceboth vested and non-vestedusing future salary levels. b. all years of serviceboth vested and...
-
Yuri Co. operates a chain of gift shops. The company maintains a defined contribution pension plan for its employees. The plan requires quarterly installments to be paid to the funding agent, Whims...

Study smarter with the SolutionInn App


