Reaction (20.16) is started with pure DTBP at 147 C and 800.0 mmHg pressure in a flask
Question:
Reaction (20.16) is started with pure DTBP at 147 °C and 800.0 mmHg pressure in a flask of constant volume.
(a) What is the value of the rate constant k?
(b) At what time will the partial pressure of DTBP be 50.0 mmHg?
Reaction (20.16)
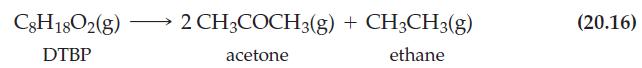
Transcribed Image Text:
C8H1802(g) DTBP 2 CH3COCH3(g) + CH3CH3(g) acetone ethane (20.16)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Analyze In part a we observe from Figure 205 that t 12 80 x 1...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer to Example 20-7. For the decomposition of di-tbutyl peroxide (DTBP), determine the time at which the total gas pressure is 2100 mmHg. Example 20-7 Reaction (20.16) is started with pure DTBP at...
-
A saturated solution of sucrose (C12H22O11) is made by dissolving excess table sugar in a flask of water. There are 50 g of un-dissolved sucrose crystals at the bottom of the flask in contact with...
-
The equilibrium reaction N 2 O 4 (g) 2 NO 2 (g) has been thoroughly studied (Figure 15.7). (a) If the total pressure in a flask containing NO 2 and N 2 O 4 gas at 25C is 1.50 atm and the value of K...
-
Data Set 14 in Appendix B lists highway fuel consumption amounts (mi/gal) for cars categorized by size (small, midsize, large). If we use those highway fuel consumption amounts arranged into the...
-
Franklin Company purchased a machine on January 1, 2008, paying $150,000. The machine was estimated to have a useful life of eight years and an estimated salvage value of $30,000. In early 2010, the...
-
What is the geometry of each species? a. CN b. PO43
-
Discuss how reality checks and discipline are involved in controlling and managing changes to the project schedule? LO.1
-
Fill in the missing blocks for the ANOVA summary table on net profits and market value used with regression analysis. a. What does the F tell you? (Alpha= .05) b. What is the t value? Explain...
-
Both GAAP and IFRS require that firms provide balance sheets and income statements from at least 2 years in annual financial reports. Why would the comparative financial statements be required?...
-
If the rate of reaction (20.3) is 5.7 x 10 4 M s 1 , what is the rate of production of O 2 (g) from 1.00 L of the H 2 O 2 (aq), expressed as (a) mol O2 s 1 ; (b) mol O 2 min 1 ; (c) mL O 2 (STP) min...
-
Use a value of k = 7.30 x 10 -4 s -1 for the first-order decomposition of H 2 O 2 (aq) to determine the percent H 2 O 2 that has decomposed in the first 500.0 s after the reaction begins.
-
If you were marketing a new sneaker, what sort of mobile applications might enhance your marketing efforts?
-
Q2 2 Points The time between students pinging professor with questions during an exam is modeled by an exponential random variable X (measured in minutes) with parameter (usual notation) Q2.1 1 Point...
-
A. Describe what the formula P = M A E represents. B. What happens if one of these factors becomes deficient? C. In terms of performance, identify the four different types of reinforcement. Provide...
-
1-3.2) K Question 10, 3.1.37 Part 1 of 6 > HW Score: 53.33%, 6.4 of 12 points O Points: 0 of 1 Save Because the mean is very sensitive to extreme values, it is not a resistant measure of center. By...
-
Research the company and obtain the following information: Mission Statement - Purpose of their existence Goals and objectives (What are they in business for) SWOT analysis for this company Based on...
-
please write one page for the concept of organizational structure one page paper of factors affecting organizational structure.
-
Describe the material properties that have an effect on the relative position of the curves shown in Fig. 7.19.
-
You are a Loan Officer with an Investment Bank. Today you need to set your lending parameters. They are: LTV: 55% 10 Year T-Bill: TBD Rate Markup: 300 Basis Points Term: 30 Years Amortization: 30...
-
The static manufacturing overhead budget based on 40,000 direct labor hours shows budgeted indirect labor costs of $54,000. During March, the department incurs $65,000 of indirect labor while working...
-
A static overhead budget based on 40,000 direct labor hours shows Factory Insurance $6,500 as a fixed cost. At the 50,000 direct labor hours worked in March, factory insurance costs were $6,200. Is...
-
Kate Coulter is confused about how a flexible budget is prepared. Identify the steps for Kate.
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


