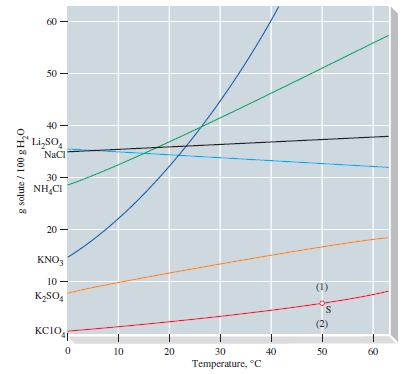
Refer to Figure 14-10 and determine the molality of NH 4 Cl in a saturated aqueous solution
Question:
Refer to Figure 14-10 and determine the molality of NH4Cl in a saturated aqueous solution at 40 °C.
Figure 14-10
Transcribed Image Text:
g solute / 100 g H₂0 50- 40 Li₂SO4 NaCl 30- NHẠC 20 KNO3 10- K₂SOA KCIO 0 10 20 30 Temperature, C 40 €0-8 50 60
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Given data From Figure1410 47g NH4Cl is dissolved in 100g water at 40 C molar ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 14-10 and estimate the temperature at which a saturated aqueous solution of KClO 4 is 0.200 m. Figure 14-10 g solute / 100 g H0 8 50- LiSO NaCl 30- NHCI 20 KNO3 10- KSO4 wow KCIO 0...
-
You just graduated and need money to buy a new car.Your rich Uncle Henry will lend you the loan as long as you agree to repay it within four years and offer to pay him the interest rate he would...
-
3.12 Suppose the spot rates of interest for investment horizons of 1 to 5 years are 4%, and for 6 to 10 years are 5%. (a) Compute the forward rates of interest if for t = 1,2, -.. ,10. (b) Calculate...
-
In which control account would you expect to find a provision for doubtful debts?
-
Pace Labs, Inc. provides mad cow disease testing for both state and federal governmental agricultural agencies. Because the companys customers are governmental agencies, prices are strictly...
-
Confidence level is 95%, is known to be $4,385,000, and the dotplot of a sample of Red Sox baseball player salaries is as shown below. Assume that we want to construct a confidence interval. Do one...
-
Examine the approaches used in defining international social responsibility
-
The controller of the South Charleston plant of Ravinia, Inc., monitored activities associated with materials handling costs. The high and low levels of resource usage occurred in September and March...
-
Little OIl has 1 million shares outstanding with a total market value of $ 2 0 million. The firm is expected to pay $ 1 million of dividends next year, and thereafter the amount paid out is expected...
-
The amount of CO 2 in the ocean is approximately 280 ppm. What is the mole fraction of CO 2 in a liter of ocean water?
-
The Environmental Protection Agency has a limit of 15 ppm for the amount of lead in drinking water. If a 1.000 mL sample of water at 20 C contains 15 ppm of lead, how many lead ions are there in this...
-
Provide a description of the history and development of the DHS.
-
Organizational conflict is the discord that arises when the goals, interests, or values of different individuals or groups are incompatible and those individuals or groups block or thwart one...
-
On October 1, 2021, Santana Rey launched a computer services company called Business Solutions, which provides consulting services, computer system installations, and custom program development. The...
-
You will identify any government regulations or laws that must be complied in your Technical Strategy and Tactical Business Proposal, along with any intellectual property that must be protected....
-
Prepare appropriate reports that will support the evaluation of data in Phase 2. With additional data provided, you are to select reports that will help address the proprietor's concerns. Some...
-
Write an R program that generates a limited exponents chart for 3, 4, and 5 (powers of 3, powers of 4,and powers of 5) and perform some calculations. 1. Write the function powerSeq(base, maxExp)...
-
The filter in Fig. 14.90(b) has a 3-dB cutoff frequency at 1 kHz. If its input is connected to a 120-mV variable frequency signal, find the output voltage at: (a) 200 Hz (b) 2 kHz (c) 10 kHz
-
A summary of changes in Pen Corporation's Investment in Sam account from January 1, 2011, to December 31, 2013, follows (in thousands): ADDITIONAL INFORMATION 1. Pen acquired its 80 percent interest...
-
Financial ratios were described in Chapter. If you were a credit manager, to which financial ratios would you pay most attention? Which do you think would be the least informative?
-
Discuss the problems with developing a numerical credit scoring system for evaluating personal loans. You can only test your system using data for applicants who have in the past been granted credit....
-
Discuss ways in which real-life decisions are more complex than the decision illustrated in figure. How do you think these differences ought to affect the credit decision? Period 1 Poriod 2...
-
I have been given topic of food bank, could you please help me what can be Resouces , events and agents in food bank revenue cycle.
-
Highmoor, a public listed company, acquired 80% of Slowmoors ordinary shares on 1 October 20x2. Highmoor paid an immediate $2 per share in cash and agreed to pay a further $1.2 per share if Slowmoor...
-
Which investment should I choose? Bond A: BBB Corporate bond, Price=$1,100, Par=$1,000, Coupon rate=4% (semiannual coupons), 13 years to maturity Bond B: BBB Corporate bond, Price=$5,900, Par=$5,000,...

Study smarter with the SolutionInn App


