Refer to Figure 9-11 and explain why the difference between the ionic radii of the -1 and
Question:
Refer to Figure 9-11 and explain why the difference between the ionic radii of the -1 and -2 anions does not remain constant from top to bottom of the periodic table.
Figure 9-11
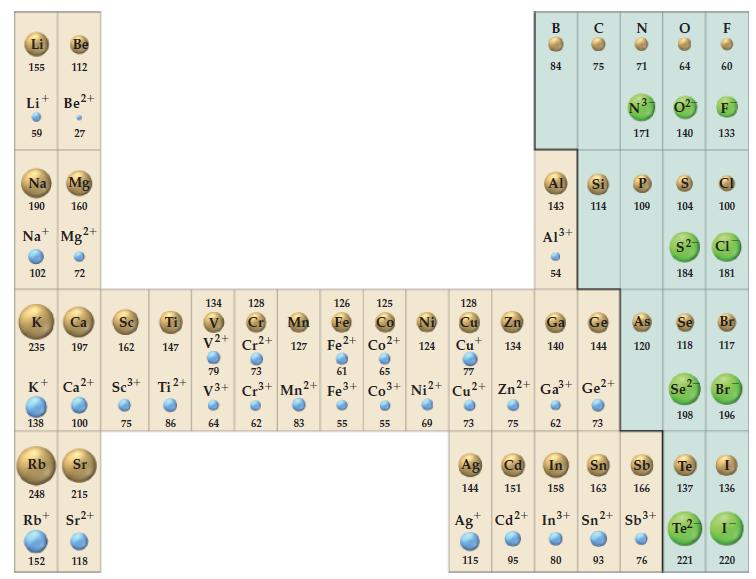
Transcribed Image Text:
Li 155 Li+ Be²+ 59 Na Mg 190 160 102 K 235 Be 112 Na Mg 138 27 Rb 2+ 152 K+ Ca²+ Sc³+ Ti ²+ 72 Ca Sc Ti 197 162 147 100 Sr 248 215 Rb Sr²+ 2+ 118 75 86 134 V V2+ 128 64 ២ Cr2+ 79 73 V3+ Cr³+ 62 Mn2+ 126 Fe Mn 127 Fe²+ Co²+ 83 61 Fe³+ 125 55 € 65 € 124 55 128 77 Co³+ Ni2+ Cu2+ Zn²+ 69 Cu+ 73 Ag 144 115 75 B 84 95 Al 143 Zn Ga 134 140 A1³+ 54 62 75 Ga³+ Ge²+ 80 144 Cd In Sn 151 158 163 Ag Cd2+ In³+ Sn 2+ 73 ZO Si P 114 109 93 N 71 N3 171 120 Sb³+ CO 76 3 64 S 0² 140 133 104 $2 184 Se 118 2- Se² Sb Te 166 137 198 Te² F 221 60 100 CI 181 Br 117 Br 196 136 I 220
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The difference between the ionic radii of the 1 and 2 anions does not remain constant from top to bo...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Explain why the difference between put and call prices depends on whether or not the underlying security pays a dividend during the life of the contracts.
-
Explain why the difference between engineering strain and true strain becomes larger as strain increases. Is this phenomenon true for both tensile and compressive strains? Explain.
-
The following figure shows the market for lowskilled labor. The value of marginal product of high-skilled workers is $16 an hour greater than that of lowskilled workers at each quantity of labor. The...
-
Voguish Couches is a small company which makes sofas. The company is owned by the couple Lennox and Stewart. You are an intern working at Voguish Couches. For the sake of simplicity, this assignment...
-
You have accumulated $8,000 and are looking for the best rate of return that can be earned over the next year. A bank savings account will pay 6%. A one-year bank certificate of deposit will pay 8%,...
-
Derive the differential equation governing the motion of the one degree-of-freedom system by applying the appropriate form(s) of Newton's laws to the appropriate free-body diagrams. Use the...
-
1. Consider the information technology skills and needs of your parents, relatives, family friends, and others in the Baby Boomer generation. Though you may not know it, you possess many skills that...
-
The T-account showing the manufacturing overhead activity for Jackson, Corp., for 2012 is as follows: Requirements 1. What is the actual manufacturing overhead? 2. What is the allocated manufacturing...
-
Hel Alyeski Tours operates day tours of coastal glaciers in Alaska on its tour boat the Blue Glacier. Management has identified two cost drivers--the number of cruises and the number of...
-
Explain why the third ionization energy of Li(g) is an easier quantity to calculate than either the first or second ionization energies. Calculate the third ionization energy for Li, and express the...
-
Two elements, A and B, have the electron configurations shown. (a) Which element is a metal? (b) Which element has the greater ionization energy? (c) Which element has the larger atomic radius? (d)...
-
Dunning College, a private college in northern Idaho, receives the following donations: 1. A parent of a current accounting student contributes $1,000,000, with the stipulation that the donation be...
-
1. How will you check if a class is a child of another class? 2. What is init method in python?
-
1. What are lists and tuples? What is the key difference between the two? 2. What is Scope in Python?
-
1. What is an Interpreted language? 2. What is a dynamically typed language?
-
Q.1 If denotes increasing order of intensity, then the meaning of the words [talk shout scream] is analogous to [please pander]. Which one of the given options is appropriate to fill the blank? (A)...
-
Consider the topology shown in Figure 4.44. Suppose that all links have unit cost and that node E is the broadcast source. Using arrows like those shown in Figure 4.44) indicate links over which...
-
Does log 81 (2401) = log 3 (7)? Verify the claim algebraically.
-
TufStuff, Inc., sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the companys products is a heavy-duty corrosion-resistant metal drum,...
-
The Scottie Sweater Company produces sweaters under the Scottie label. The company buys raw wool and processes it into wool yarn from which the sweaters are woven. One spindle of wool yam is required...
-
Variable costs and differential costs mean the same thing. Do you agree? Explain.
-
A company purchased $3,400 of merchandise on July 5 with terms 3/10, n/30. On July 7, it returned $600 worth of merchandise. On July 8, it paid the full amount due. The amount of the cash paid on...
-
A corporation that incurs a net operating loss may carry the loss back to earlier years before it can carry the loss forward. True or False
-
Question 2 Prepare the journal entries to record the following transactions on Ivanhoe Company's books using a perpetual inventory system. (If no entry is required, select "No Entry" for the account...

Study smarter with the SolutionInn App


