Show that in terms of mole fractions of gases and total gas pressure the equilibrium constant expression
Question:
Show that in terms of mole fractions of gases and total gas pressure the equilibrium constant expression for
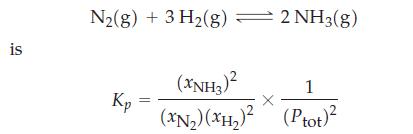
Transcribed Image Text:
is N₂(g) + 3 H₂(g) = 2 NH3(g) Kp (XNH3)² 1 (XN₂) (XH₂)² (Ptot)²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The equilibrium constant expression for a gasphase reaction can be written in terms of either partia...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Methane (CH 4 ) gas flows into a combustion chamber at a rate of 200. L/ min at 1.50 atm and ambient temperature. Air is added to the chamber at 1.00 atm and the same temperature, and the gases are...
-
A continuous distillation unit, consisting of a perforated-tray column together with a partial reboiler and a total condenser, is to be designed to operate at atmospheric pressure to separate ethanol...
-
Write the equilibrium constant expression for the dissolution of ammonia in water: Use this equilibrium constant expression to estimate the partial pressure of NH 3 (g) over a solution containing 5 x...
-
Olmsted Co. has small computer chips assembled in Poland and transports the final assembled products to the parent, where they are sold by the parent in the U.S. The assembled products are invoiced...
-
Columbia Paper has the following stockholders equity account. The firms common stock has a current market price of $30 per share. Preferred stock .............$100,000 Common stock (10,000 shares at...
-
What is an adjustable rate mortgage?
-
8. An electronics conglomerate intends to divest its high-growth energy division, which develops and manufactures solar panels, windmills, and other green energy products. The division has an...
-
Safety Stocks and Order Points Sache, Inc expects to sell 700 of its designer suits every week. The store is open seven days a week and expects to sell the same number of suits every day. The company...
-
Bill's car was totally destroyed during a federally declared disaster in 2020. The car had a fair market value of $18,000 before it was destroyed and cost him $16,000 when he purchased it two years...
-
A mixture of H 2 S(g) and CH 4 (g) in the mole ratio 2 : 1 was brought to equilibrium at 700 C and a total pressure of 1 atm. On analysis, the equilibrium mixture was found to contain 9.54 x 10 -3...
-
What is the apparent molar mass of the gaseous mixture that results when COCl 2 (g) is allowed to dissociate at 395 C and a total pressure of 3.00 atm? Think of the apparent molar mass as the molar...
-
Compute the equivalent single rate of discount for each of the following discount series. (a) 30%, 12.5% (b) 33 1/3%, 20%, and 3%
-
Machine-hours required to support estimated production Fixed manufacturing overhead cost Variable manufacturing overhead cost per machine-hour Required: 1. Compute the plantwide predetermined...
-
Assume now that a new firm (firm N) discovers and patents a more efficient technology, summarized by thetotal cost function C = 10q. The new technology can be used only by the new firm, which enters...
-
1. How has Dell used virtual integration to become an industry leader? Dell has used virtual integration to become an industry leader by leveraging its global suppliers to reduce costs and provide...
-
3) Consider the asset pricing model with uncertainty in the slide. We derived the asset prices as Pb = Ps = - [nu' (y+Yn + e) + (1 )u' (y + y + e)] u'(e1) [nu' (y +n + ) + (1 )u' (y + y + e2)] u'(e1)...
-
Amazon is considered a leader in managing its supply chain. Describe in detail two parts of Amazon's Supply Chain Management that you see as critical to their success. Please provide your reasoning...
-
On March 1, 2017, Boyd Company acquired real estate, on which it planned to construct a small office building, by paying $80,000 in cash. An old warehouse on the property was demolished at a cost of...
-
The process of collaborative goal setting by a manager and subordinate, the extent to which goals are accomplished is a major factor in evaluating and rewarding the subordinate's performance. It is...
-
Washington Co. operates a chain of bookstores. The company maintains a defined contribution pension plan for its employees. The plan requires quarterly installments to be paid to the funding agent,...
-
In a recent years financial statements, Procter & Gamble showed an unfunded pension liability of $2,637 million and a periodic pension cost of $183 million. Explain the meaning of the $2,637 million...
-
Lachgar Industries warrants its products for one year. The estimated product warranty is 4% of sales. Assume that sales were $210,000 for June. In July, a customer received warranty repairs requiring...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


