Sketch a titration curve (pH versus mL of titrant) for each of the following three hypothetical weak
Question:
Sketch a titration curve (pH versus mL of titrant) for each of the following three hypothetical weak acids when titrated with 0.100 M NaOH. Select suitable indicators for the titrations from Figure 17-7.
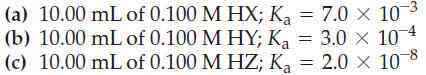
Figure 17-7
Transcribed Image Text:
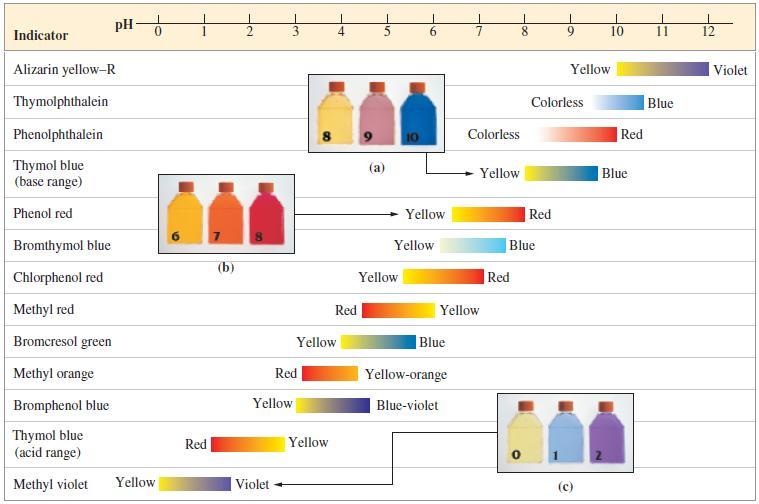
(a) 10.00 mL of 0.100 M HX; Ka = 7.0 x 10 (b) 10.00 mL of 0.100 M HY; Ka = 3.0 x 10-4 (c) 10.00 mL of 0.100 M HZ; K₂ = 2.0 × 10 Ka
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Sketch a titration curve (pH versus mL of titrant) for each of the following hypothetical weak bases when titrated with 0.100 M HCl. (Think of these bases as involving the substitution of organic...
-
At times, a salt of a weak base can be titrated by a strong base. Use appropriate data from the text to sketch a titration curve for the titration of 10.00 mL of 0.0500 M C 6 H 5 NH 3 + Cl - with...
-
Hrishi is a senior executive for a large manufacturing company in Mississauga Ontario where he has been employed for the past 10 years and his annual salary is $250,000 including bonus. He is 47...
-
Tantor Supply, Inc., is a small corporation acting as the exclusive distributor of a major line of sporting goods. During 2010 the firm earned $92,500 before taxes. a. Calculate the firm?s tax...
-
The table shows monthly time-series data for short-term visits to the United Kingdom and its territories by Australian residents. (a) Explore trends in these data by using linear and quadratic trend...
-
What rules will apply to the process of negotiations?
-
Ceramic Painting prepares and packages paint products. Ceramic Painting has two departments: Blending and Packaging. Direct materials are added at the beginning of the blending process (dyes) and at...
-
Oriole Company is a small restaurant that sells a variety of sandwiches and beverages. Total fixed costs are $19,300 per month. Last month, total variable costs were $6,700 when total sales were...
-
Determine the following characteristics of the titration curve for 20.0 mL of 0.275 M NH 3 (aq) titrated with 0.325 M HI(aq). (a) The initial pH; (b) The volume of 0.325 M HI(aq) at the equivalence...
-
Explain whether the equivalence point of each of the following titrations should be below, above, or at pH 7: (a) NaHCO 3 (aq) titrated with NaOH(aq); (b) HCl(aq) titrated with NH 3 (aq); (c) KOH(aq)...
-
Create a flowchart from the following sequence of activities: Begin. Flow to activity A. Flow to decision B. If Yes, flow to activity C. If No, flow to activity D. From C flow to activity E and to...
-
Requirement 1. Prepare a horizontal analysis of the comparative income statement of McCormick Designs, Inc. Round percentage changes to one decimal place. (Round the percentages to one decimal place,...
-
GAAP looks to compare entities with an apples-to-apples valuation so that the entities can be viewed by interested parties to compare financial values and how effectively and efficiently they use...
-
Prepare a statement of cash flows for Wu using the indirect method.
-
A plate of steel with a central through-thickness flaw of length 16 mm is subjected to a stress of 350 MPa normal to the crack plane. If the yield strength of the material is 1400 MPa what is the...
-
More info The company manufactures a variety of engines for use in farm equipment. At the beginning of the current year, Dansville estimated that its overhead for the coming year would be $300,000....
-
Suppose the composite bar in Exercise 11.1.8 is only fixed at its top end and subject to a uniform gravitational force. Will the free end hang lower if its stiffer half is on top or bottom? Make a...
-
Which provision could best be justified as encouraging small business? a. Ordinary loss allowed on $ 1244 stuck. b. Percentage depletion. c. Domestic production activates deductions. d. Interest...
-
From the following list, identify the accounts that should be closed to Income Summary at the end of the fiscal year: a. Accounts Receivable b. Accumulated DepreciationEquipment c. Depreciation...
-
Prior to its closing, Income Summary had total debits of $432,200 and total credits of $572,600. Briefly explain the purpose served by the income summary account and the nature of the entries that...
-
After all revenue and expense accounts have been closed at the end of the fiscal year, Income Summary has a debit of $193,400 and a credit of $258,600. At the same date, Laurie Engan, Capital has a...
-
(International Finance) Computing a Currency changes = (e1 - e0 )/ e0 where e0 = old currency value e1 = new currency value (a) If the dinar devalues against the U.S. dollar by 45%, the U.S. dollar...
-
why should Undertake research to review reasons for previous profit or loss?
-
Use the failure probability and consequence scores shown in the table to determine the risk factor for the project. Maturity 0.2 Cost 0.5 Complexity 0.5 Schedule 0.1 Dependency 0.4 Reliability 0.2...

Study smarter with the SolutionInn App


