Sodium hydrogen sulfate, NaHSO 4 , is an acidic salt with a number of uses, such as
Question:
Sodium hydrogen sulfate, NaHSO4, is an acidic salt with a number of uses, such as metal pickling (removal of surface deposits). NaHSO4 is made by the reaction of H2SO4 with NaCl. To determine the percent NaCl impurity in NaHSO4, a 1.016 g sample is titrated with NaOH(aq); 36.56 mL of 0.225 M NaOH is required.
(a) Write the net ionic equation for the neutralization reaction.
(b) Determine the percent NaCl in the sample titrated.
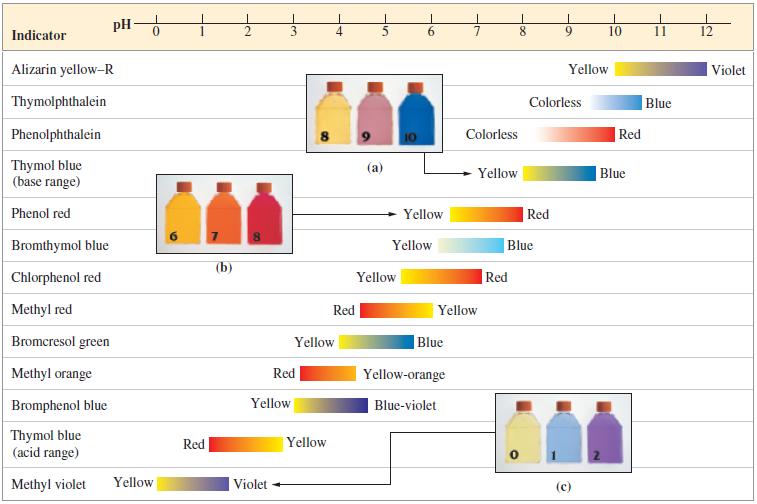
(c) Select a suitable indicator from Figure 17-7.
Figure 17-7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





