This single equilibrium equation applies to different phenomena described in this or the preceding chapter. CH3COOH +
Question:
This single equilibrium equation applies to different phenomena described in this or the preceding chapter.
Transcribed Image Text:
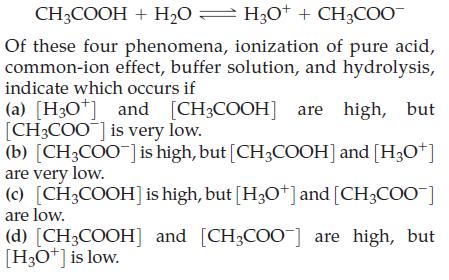
CH3COOH + H₂0 H3O+ + CH3COO Of these four phenomena, ionization of pure acid, common-ion effect, buffer solution, and hydrolysis, indicate which occurs if (a) [H3O+] and [CH3COOH] are high, but [CH3COO] is very low. (b) [CH3COO] is high, but [CH3COOH] and [H3O+] are very low. (c) [CH3COOH] is high, but [H3O+] and [CH3COO-] are low. (d) [CH3COOH] and [CH3COO] are high, but [H3O+] is low.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The given equilibrium equation is CH3COOH HO HO CH3COO Lets analyze each scenario a If H3O and CH3CO...View the full answer

Answered By

Tamil Elakkiya Rajendran
I'm currently involved in the research in the field of Biothermodynamics, Metabolic pathway analysis and computational Biology. I always prefer to share my knowledge whatever I have learnt through my degree whenever time permits.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Based on Westlaw's data on the case Christoff v. Nestl USA, INC., I did the brief case. My question is, is there any need to add and adjust the content and form of the case brief, especially the...
-
:{"foster", "enthusiasm", "wagon", "ally", "lehigh", "programming", "dog", "cat", "Ally", "smile", "pet" }; a. Suppose you perform insertion sort in order to sort A in ascending order. How many...
-
The overall goal of this problem is to compute the PV and PT equilibrium diagramsfor a single component fluid described by the van derWaals equation of state. Let us recall the key things we need to...
-
obias is a foreman at a factory that manufactures auto parts. When a health a safety inspector visits the plant, Tobias notices the inspector writing down several major labor and safety violations....
-
To assess the firms average collection period and average payment period ratios, what additional information is needed, and why?
-
Prepare journal entries for each transaction listed. a. During the period, customer balances are written off in the amount of $17,000. b. At the end of the period, bad debt expense is estimated to be...
-
Does the organization want applicants who are different from the companys current employees?
-
Record the following process costing transactions in the general journal: a. Purchase of raw materials on account, ............ $9,000 b. Requisition of direct materials to Assembly Department,...
-
The following information is from DALLAS Manufacturing Co. for April: $95,000 $76,000 $234,000 $32,000 Direct materials used in production Direct labour Total manufacturing cost Raw materials...
-
Sodium hydrogen sulfate, NaHSO 4 , is an acidic salt with a number of uses, such as metal pickling (removal of surface deposits). NaHSO 4 is made by the reaction of H 2 SO 4 with NaCl. To determine...
-
What stoichiometric concentration of the indicated substance is required to obtain an aqueous solution with the pH value shown: (a) Aniline, C 6 H 5 NH 2 , for pH = 8.95; (b) NH 4 Cl for pH = 5.12?
-
Using the information in question 4, explain to management the possible reasons multiple markdowns could have been taken and why we compare to plan.
-
6. [-/2.94 Points] DETAILS WANEFMAC8 12.2.037.EP. MY NOTES ASK YOUR TEACHER 1,500 The demand equation for your company's virtual reality video headsets is p = where q is the total number of headsets...
-
V W R + |, V W R R3 w V3 V In the above circuit diagram, if V=12V, R =42, R2=62, R3=12.2. What would be the value of current I? (Hint: use Kirchhoff's law) 2 0.5A 1.5A 1A 2A
-
A company produces a single product that has the following standard costs: Materials Labor 5 pieces at P4 per piece P20 3 hours at P10 per hour 30 Variable Overhead 3 hours at P15 per hour 45 Fixed...
-
Use the graph to answer each part. K (a) List all the even vertices and all the odd vertices. Click on "None" as needed. List of the even vertices: List of the odd vertices: (b) List all vertices...
-
My software development company has the databases, tools, and staff to design up to 24 programs per week with an effective capacity of 17 programs per week. What is its efficiency if it designs an...
-
Explain in detail why there is no continuous function y(x) such that the inner product identity (11.39) holds for every continuous function u(x).
-
Refrigerant-134a enters an adiabatic compressor as saturated vapor at 120 kPa at a rate of 0.3 m3/min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 80 percent,...
-
During the month, Genesis Labs Co. has a substantial number of transactions affecting each of the following accounts. State for each account whether it is likely to have (a) Debit entries only, (b)...
-
The following table summarizes the rules of debit and credit. For each of the items (a) through (l), indicate whether the proper answer is a debit or acredit. Normal Balance Increase Decrease Balance...
-
As of January 1, Oh Kwon, Capital, had a credit balance of $37,100. During the year, withdrawals totaled $1,000, and the business incurred a net loss of $52,300. a. Calculate the balance of Oh Kwon,...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Describe several common sources of yardstick data that you can use as bases for developing forecast assumptions
-
There are six farmers in Great Britain with access to government land to graze their cows for free. They all must share the land. Each farmer has an individual incentive to put as many of his cows on...

Study smarter with the SolutionInn App


