Some electrochemical cells employ large biological molecules known as enzymes. An enzyme increases the rate of a
Question:
Some electrochemical cells employ large biological molecules known as enzymes. An enzyme increases the rate of a biochemical reaction. Some enzymes perform oxidation reactions, and some others perform reduction reactions. An electrochemical cell based on the use of enzymes is given in the following illustration.
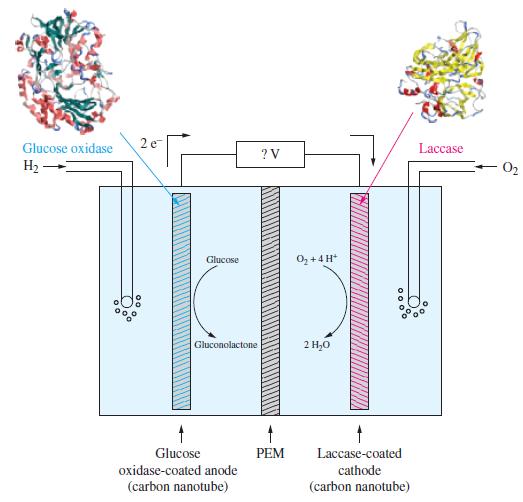
The half-cell reactions for electrochemical cell and the corresponding reduction potentials are given below. The symbol E°’ is used here because these values refer to the biological standard state: 37 °C, aH+ = 10-7, all other activities equal to 1.
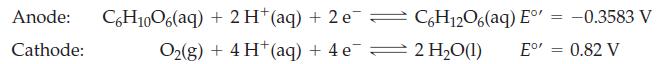
(a) Write an equation for the overall reaction occurring in this electrochemical cell.
(b) Write a cell diagram for this battery.
(c) Calculate the standard cell potential for the electrochemical reaction.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





