The accompanying illustration shows a 100.0 mL graduated cylinder half-filled with 8.0 g of diatomaceous earth, a
Question:
The accompanying illustration shows a 100.0 mL graduated cylinder half-filled with 8.0 g of diatomaceous earth, a material consisting mostly of silica and used as a filtering medium in swimming pools. How many milliliters of water are required to fill the cylinder to the 100.0 mL mark? The diatomaceous earth is insoluble in water and has a density of 2.2 g/cm3. 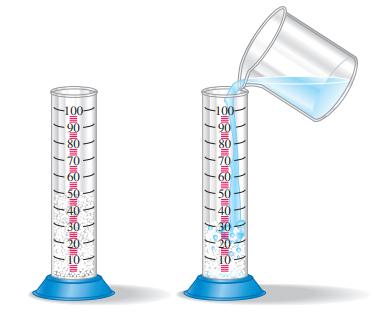
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





