The combustion of methane gas, the principal constituent of natural gas, is represented by the equation (a)
Question:
The combustion of methane gas, the principal constituent of natural gas, is represented by the equation
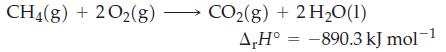
(a) What mass of methane, in kilograms, must be burned to liberate 2.80 x 107 kJ of heat?
(b) What quantity of heat, in kilojoules, is liberated in the complete combustion of 1.65 x 104 L of CH4(g), measured at 18.6 °C and 768 mmHg?
(c) If the quantity of heat calculated in part (b) could be transferred with 100% efficiency to water, what volume of water, in liters, could be heated from 8.8 to 60.0 °C as a result?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





