The Deacon process for producing chlorine gas from hydrogen chloride is used in situations where HCl is
Question:
The Deacon process for producing chlorine gas from hydrogen chloride is used in situations where HCl is available as a by-product from other chemical processes.
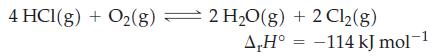
A mixture of HCl, O2, H2O, and Cl2 is brought to equilibrium at 400 °C. What is the effect on the equilibrium amount of Cl2(g) if
(a) Additional O2(g) is added to the mixture at constant volume?
(b) HCl(g) is removed from the mixture at constant volume?
(c) The mixture is transferred to a vessel of twice the volume?
(d) A catalyst is added to the reaction mixture?
(e) The temperature is raised to 500 °C?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





