The electrolysis of MgCl 2 (aq) can be represented as The electrolysis of a 315 mL sample
Question:
The electrolysis of MgCl2(aq) can be represented as
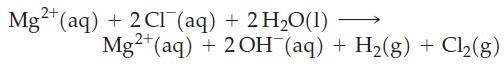
The electrolysis of a 315 mL sample of 0.185 M MgCl2 is continued until 0.652 L H2(g) at 22 °C and 752 mmHg has been collected. Will Mg(OH)2(s) precipitate when electrolysis is carried to this point? Notice that [Mg2+] remains constant throughout the electrolysis, but [OH¯] increases.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





