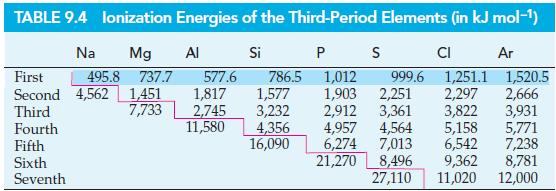
The first ionization energies of Si, P, S, and Cl are given in Table 9.4. Briefly provide
Question:
The first ionization energies of Si, P, S, and Cl are given in Table 9.4. Briefly provide an explanation for this trend.
Table 9.4
Transcribed Image Text:
TABLE 9.4 lonization Energies of the Third-Period Elements (in kJ mol-¹) Ar Si Na Mg First 495.8 737.7 Second 4,562 1,451 7,733 Third Fourth Fifth Sixth Seventh Al 577.6 1,817 2,745 11,580 786.5 1,577 3,232 4,356 16,090 PS 1,012 1,903 2,251 2,912 3,361 4,957 4,564 6,274 7,013 21,270 999.6 CI 1,251.1 2,297 2,666 3,822 3,931 1,520.5 5,158 5,771 6,542 7,238 8,496 9,362 8,781 27,110 11,020 12,000
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The trend in the first ionization energies of Si P S and Cl is that they increase as you move from l...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The accompanying graphs show the first ionization energies and electron affinities of the period 3 elements. Refer to the graphs to answer the questions that follow. a. Describe the general trend in...
-
An alternative definition of electronegativity is Electronegativity 5 constant (I. E. 2 E. A.) where I. E. is the ionization energy and E. A. is the elec-tron affinity using the sign conventions of...
-
The first ionization energies of As and Se are 0.947 MJ/ mol and 0.941 MJ/mol, respectively. Rationalize these values in terms of electron configurations.
-
Preparing and interpreting a statement of cash flows using a T-account work sheet. Financial statement data for Dickerson Manufacturing Company for the current year appear in Exhibit 5.29. Additional...
-
Circle-Square, Ltd., is in the process of liquidating and going out of business. The firms balance sheet shows $22,800 in cash, accounts receivable of $114,200, inventory totaling $61,400, plant and...
-
How do embedded real options affect the values of investment opportunities? Why, when assessing investment alternatives, is it important to consider the embedded real options?
-
Q6 What is a VPN, and why is it important?
-
Transaction Analysis Pollys Cards & Gifts Shop had the following transactions during the year: a. Pollys purchased inventory on account from a supplier for $8,000. Assume that Pollys uses a periodic...
-
Jane has taken a part-time job to save for a $1180 racing bicycle. If she puts $65 each week into a savings account paying 5.2% compounded weekly, how many weeks will she be able to buy the racing...
-
Describe how the ionization energies of the ions Be + , B + , C + , N + , O + , F + , Ne + , and Na + vary with atomic number.
-
Which element Na or Mg is likely to have ea H greater than zero?
-
The population of the US was 281.4 million in 2000 and 316.1 million in 2013.103 Assuming exponential growth, (a) In what year is the population expected to go over 350 million? (b) What population...
-
Let two planes be given by 2x-y+z = 8 and z = x+y-5 (a) Find the angle between the two planes. Leave your answer in degrees and round to the nearest tenth. (b) Find the vector equation of the line of...
-
9-2. The profile of a gear tooth shown in Fig. P9.2 is approximated by the trigonometric equation y(x) = a. Estimate the area A using eight rectangles of equal width A x = 1/8, b. Calculate the exact...
-
tube is hinged to a rotating base as shown in Fig. 4. At the instant shown, the base rotates about the z axis with a constant angular velocity ! 1 = 2 rad/s. At the same instant, the 2 tube rotates...
-
Find the limit analytically. -7x2+5x-10 lim 0 9x+13x+11 Find the limit analytically. lim +80 4x-13 5x+6x-11
-
Write a recursive function for the running time T(n) of the function given below. Prove using the iterative method that T(n) = (n). function( int n) { if(n=1) return; for(int i = 1; i
-
A bare-bones forwarding table in a VC network has four columns. What is the meaning of the values in each of these columns'? A bare-bones forwarding table in a datagram network has two columns. What...
-
A copper wire (density = 8.96 g/cm 3 ) has a diameter of 0.25 mm. If a sample of this copper wire has a mass of 22 g, how long is the wire?
-
Polaski Company manufactures and sells a single product called a Ret. Operating at capacity, the company can produce and sell 30,000 Rets per year. Costs associated with this level of production and...
-
Come-Clean Corporation produces a variety of cleaning compounds and solutions for both industrial and household use. While most of its products are processed independently, a few are related, such as...
-
Losses have been incurred at Millard Corporation for some time. In an effort to isolate the problem and improve the companys performance, management has requested that the monthly income statement be...
-
Suppose your firm is considering investing in a project with the cash flows shown below, that the required rate of return on projects of this risk class is 8 percent, and that the maximum allowable...
-
A credit union entered a lease contract valued at $ 6100. The contract provides for payments at the end of each month for 8 years. If interest is 4 % compounded quarterly, determine the present value...
-
f(x) - J cincidit f(x) - J cincidit

Study smarter with the SolutionInn App


