The ionization energies of Li, Be + , B 2+ , and C 3+ are, respectively, 520,
Question:
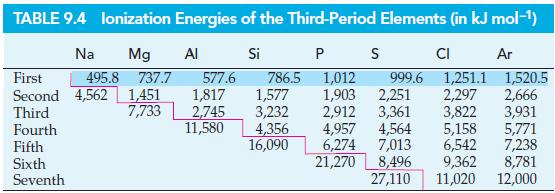
The ionization energies of Li, Be+, B2+, and C3+ are, respectively, 520, 1757, 3659, and 6221 kJ mol–1. The ionization energies Na, Mg+, Al2+, and Si3+ are (from Table 9.4) 495.8, 1451, 2745, and 4356 kJ mol–1. Plot a graph of the square roots of the ionization energies versus the nuclear charge for these two series. Explain the observed relationship with the aid of Bohr’s expression for the binding energy of an electron in a one-electron atom.
Table 9.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





