The pH of ocean water depends on the amount of atmospheric carbon dioxide. The dissolution of carbon
Question:
The pH of ocean water depends on the amount of atmospheric carbon dioxide. The dissolution of carbon dioxide in ocean water can be approximated by the following chemical reactions (Henry’s Law constant for CO2 is KH = [CO2(aq)]/[CO2(g)] = 0.8317.) For reaction (2), K = 2.8 x 10-9:
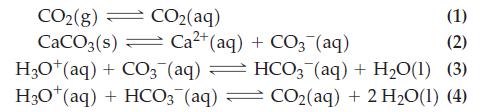
(a) Use the equations above to determine the hydronium ion concentration as a function of [CO2(g)] and [Ca2+].
(b) During preindustrial conditions, we will assume that the equilibrium concentration of [CO2(g)] = 280 ppm and [Ca2+] = 10.24 mM. Calculate the pH of a sample of ocean water.
Transcribed Image Text:
CO2(g) — CO2(aq) (1) CaCO3(s) Ca²+ (aq) + CO3(aq) (2) H3O+ (aq) + CO3(aq) — HCO3(aq) + H₂O(1) (3) H3O+ (aq) + HCO3(aq) CO₂(aq) + 2 H₂O(1) (4) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
a Determine the hydronium ion concentration as a function of CO2g and Ca2 From equation 1 we can exp...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
THE DATA PROJECTOR MARKET Alset recognises that mini data projector demand is growing rapidly, although actual numbers are difficult to obtain. The latest figures show the market is $13 billion...
-
A glass of water initially at pH 7.0 is exposed to dry air at sea level at 20C. Calculate the pH of the water when equilibrium is reached between atmospheric CO2 and CO2 dissolved in the water, given...
-
Listed are the equity sections of balance sheets for years 2011 and 2012 as reported by Mountain Air Ski Resorts, Inc. The overall value of stockholders equity has risen from $2,000,000 to...
-
Industrial Holdings Inc. (IH), a public company, has been a client of Walker LLP for several years. Walker has completed the integrated audit of IH for the year ended December 31, 2022. In February...
-
What would you have predicted for the acceptance or rejection of the incentive compensation scheme at Canon in Japan? As multinational firms open more and more operations in more and more countries,...
-
Bart James, a partner in the CPA firm of James and Day, received the following memorandum from John Gray, president of Gray Manufacturing Corporation, an audit client of many years. Dear Bart: I have...
-
You are required to use the information in the table above to compute the following: a) Use four metheds to calcalate the ending Inventory, and cost of goods soid under each method. b) Prepare a...
-
In 1922 Donald D. van Slyke (J. Biol. Chem., 52, 525) defined a quantity known as the buffer index: = dc b/ d(pH), where d cb represents the increment of moles of strong base to one liter of the...
-
Avery common buffer agent used in the study of biochemical processes is the weak base TRIS, (HOCH 2 ) 3 CNH 2 , which has a pK b of 5.91 at 25 C. A student is given a sample of the hydrochloride of...
-
Sky Time Media Corporations only temporary difference at the beginning and end of 2014 is caused by a $2.5 million litigation accrual that is expected to be settled in 2016. The related deferred tax...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Receivable Sales Revenue Equipment Common Stock Notes Payable Retained Earnings...
-
Smart Sports is also planning to launch a range of drinks products. The products have been developed by Hydration Labs Ltd and are designed to be sold as powders that dissolve easily in water. They...
-
Baucom Company accepted credit cards in payment for \(\$ 6,850\) of services performed during March 2011. The credit card company charged Baucom a 4 percent service fee. The credit card company paid...
-
The following post-closing trial balance was drawn from the accounts of Spruce Timber Co. as of December 31, 2011. Transactions for 2012 1. Acquired an additional \(\$ 10,000\) cash from the issue of...
-
Bankers Trust (BT) was one of the most powerful and profitable banks in the world in the early 1990s. Under the stewardship of chairman Charles Sanford Jr., it had transformed itself from a staid...
-
(a) Determine the adjoint of the differential operator u = L[u] = u' + 2xu with respect to the L2 inner products on [0, I] when subject to the fixed boundary conditions u(0) = u(1) = 0. (b) Is the...
-
Explain the circumstances that could result in a long-term bank loan being shown in a statement of financial position as a current liability.
-
We consider the random process St, which plays a fundamental role in BIack-Scholes analyses: St = S0e[1+Wt] Where Wt is a Wiener process with W0 = 0, is a trend factor, and (Wt Ws) N(0, (I s)),...
-
Show that as n ? ? (a) 1(1 - ).-(1 (b) (1 - )" - . (e) (1-) -1
-
Let the random variable Xn have a binomial distribution: Where each Bi is independent and i distributed according to We can look at X n as the cumulated sum of a series of events that occur over...
-
The following information was available for the year ended December 31, 2022: Net sales $ 300,000 Cost of goods sold 210,000 Average accounts receivable for the year 15,000 Accounts receivable at...
-
Jeannie is an adjunct faculty at a local college, where she earned $680.00 during the most recent semimonthly pay period. Her prior year-to-date pay is $18,540. She is single and has one withholding...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...

Study smarter with the SolutionInn App


