Question: The reaction C 2 H 5 I + OH - C 2 H 5 OH + I - was studied in an ethanol (C
The reaction C2H5I + OH- → C2H5OH + I- was studied in an ethanol (C2H5OH) solution, and the following rate constants were obtained: 15.83 °C, k = 5.03 x 10-5; 32.02 °C, 3.68 x 10-4; 59.75 °C, 6.71 x 10-3; 90.61 °C, 0.119 M-1 s-1.
(a) Determine Ea for this reaction by a graphical method.
(b) Determine Ea by the use of equation (20.22).
(c) Calculate the value of the rate constant k at 100.0 °C.
Eq. 20.22
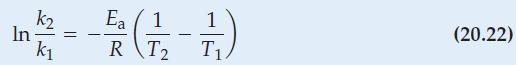
k In = - k Ea F2 (17) - RT2 T (20.22)
Step by Step Solution
3.47 Rating (150 Votes )
There are 3 Steps involved in it
a Determine Ea for this reaction by a graphical method To determine Ea for this reaction by a graphi... View full answer

Get step-by-step solutions from verified subject matter experts


