The reduction of aldehydes and ketones with a suitable hydride-containing reducing agent is a good way of
Question:
The reduction of aldehydes and ketones with a suitable hydride-containing reducing agent is a good way of synthesizing alcohols. This approach would be even more effective if, instead of a hydride, we could use a source of nucleophilic carbon. Attack by a carbon atom on a carbonyl group would give an alcohol and simultaneously form a carbon-to-carbon bond. How can we make a C atom in an alkane nucleophilic? This was achieved by Victor Grignard, who created the organometallic reagent R—MgBr, with the following reaction in diethyl ether:
![]()
The Grignard reagent is rarely isolated. It is formed in solution and used immediately in the desired reaction. The alkylmetal bond is highly polar, with the partial negative charge on the C atom, which makes the C atom highly nucleophilic. The Grignard reagent (R—MgBr) can attack a carbonyl group in an aldehyde or ketone as follows:
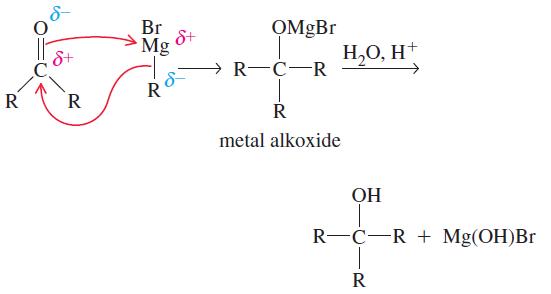
Addition of dilute aqueous acid solution to the metal alkoxide furnishes the alcohol. The important synthetic consequence of this procedure is that we have prepared a product with more carbon atoms than present in the starting material. A simple starting material can be transformed into a more complex molecule.
(a) What is the product of the reaction between methanal and the Grignard reagent formed from 1-bromobutane after the addition of dilute acid?
(b) By using a Grignard reagent, devise a synthesis for hexan-2-ol.
(c) By using a Grignard reagent, devise a synthesis for 2-methylhexan-2-ol.
(d) Grignard reagents can also be formed with aryl halides, such as chlorobenzene. What would be the product of the reaction between the Grignard reagent of chlorobenzene and propanone? Can you think of an alternative synthesis of this product, again using a Grignard reagent?
(e) The basicity of the C atom bound to the magnesium in the Grignard reagent can be used to make Grignard reagents of terminal alkynes. Write the equation of the reaction between ethylmagnesium bromide and hex-1-yne.
(f) By using a Grignard reagent, suggest a synthesis for hept-2-yn-1-ol.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





