Starting with benzene and methane, and suitable inorganic reagents, suggest how the following compound might be synthesized.
Question:
Starting with benzene and methane, and suitable inorganic reagents, suggest how the following compound might be synthesized. See Exercise 37 for a description of how an alkyl group can be added to the benzene ring.
Exercise 37
Alkylation of benzene can be accomplished by treating benzene with haloalkane (RX) in the presence of AlCl3. The reaction is known as a Friedel–Crafts alkylation reaction. (The reaction is named after Charles Friedel, a French chemist, and James M. Crafts, an American chemist, who discovered this method of making alkylbenzenes in 1877.) An example of a Friedel–Crafts alkylation reaction is shown below:
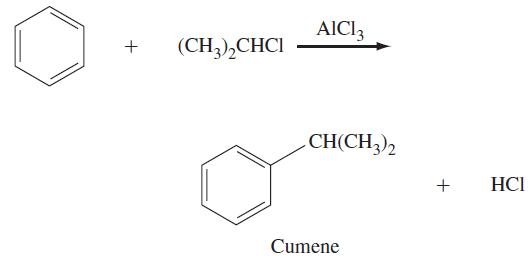
The mechanism for this reaction involves the following steps. First, (CH3)2CHCl and AlCl3 react in a Lewis acid–base reaction to form an adduct, (CH3)2CH—Cl—AlCl3, in which a chlorine atom is bonded to both carbon and aluminum. The adduct then dissociates to (CH3)2CH+ a carbocation, and AlCl4–.The carbocation acts as an electrophile in a reaction with benzene, forming an arenium ion. Finally, a proton is removed from the arenium ion by AlCl4–, yielding an alkylbenzene, HCl, and AlCl3. Write chemical equations for the elementary processes involved in forming cumene and HCl from (CH3)2CHCl and benzene. Use curved arrows to show the movement of electrons.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





