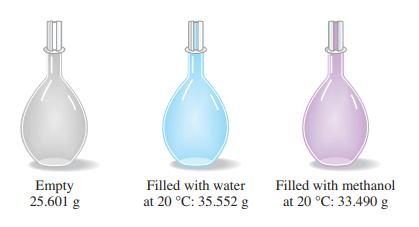
The simple device pictured here, a pycnometer, is used for precise density determinations. From the data presented,
Question:
The simple device pictured here, a pycnometer, is used for precise density determinations. From the data presented, together with the fact that the density of water at 20 °C is 0.99821 g/mL, determine the density of methanol, in grams per milliliter.
Transcribed Image Text:
Empty 25.601 g Filled with water at 20 °C: 35.552 g Filled with methanol at 20 °C: 33.490 g
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
for methanol massg33490 g and metha...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A pycnometer (see Exercise 78) weighs 25.60 g empty and 35.55 g when filled with water at 20 C. The density of water at 20 C is 0.9982 g/mL. When 10.20 g of lead is placed in the pycnometer and the...
-
If the pycnometer of Exercise 78 is filled with ethanol at 20 C instead of methanol, the observed mass is 33.470 g. What is the density of ethanol? How precisely could you determine the composition...
-
A nonconducting container tilled with 25 kg of water at 20 C is fitted with a stirrer, which is made to turn by gravity acting on a weight of mass 35 kg. The weight falls slowly through a distance of...
-
If Converse introduced a new SMART WATCH for Men and Women, should they launch the product using Market Skimming or Marketing Penetration? Explain answer
-
How can the adherence to high standards of ethical business practice contribute to the goal of shareholder wealth maximization?
-
1. How did Hennessys background prepare him for starting a business? What entrepreneurial qualities does he embody? 2. What were Hennessys entrepreneurial motivations for founding Dash-Locker? 3....
-
Describe the characteristics and differences between qualitative, quantitative, extrinsic, and intrinsic forecasting techniques.
-
The price drivers pay for gasoline often varies a great deal across regions throughout the United States. The following data show the price per gallon for regular gasoline for a random sample of...
-
Examine the Equity section in the Statement of changes in Equity and the Statement of Financial Position of your company for the 3 years and write a report containing: 1. What types of shares issued...
-
The Greater Vancouver Regional District (GVRD) chlorinates the water supply of the region at the rate of 1 ppm, that is, 1 kilogram of chlorine per million kilograms of water. The chlorine is...
-
A Fahrenheit and a Celsius thermometer are immersed in the same medium. At what Celsius temperature will the numerical reading on the Fahrenheit thermometer be (a) 49 less than that on the Celsius...
-
Three masses are suspended vertically by a series of identical springs where mass 1 is at the top and mass 3 is at the bottom. If g = 9.81 m/s2, m1 = 2 kg, m2 = 3 kg, m3 = 2.5 kg, and the k's = 10...
-
In this scenario you are the Manager of a Home and Community Care organization in Victoria. Your organization provides oversees support services for individuals in their homes. The individuals that...
-
find the wind speed and direction for the following parcels. Important note: To determine the arc tangent using the Google calculator, click the box marked "Inv" so that it is a light gray instead of...
-
As consumers we're connected to an instant feed or live updates, breaking news, and messages. We believe that when we post something on social media, we will get instant feedback from friends. What...
-
Describe the process for screening candidates for ethics. Outline which job candidate factors are illegal to consider when hiring. Explain how to obtain accurate behavior information from resumes,...
-
In relation to Operation management, sustainability and supply chain management explain the following terms; What is the overall objective of scheduling? What is JIT? What is a Lean producer? Discuss...
-
A fatty acid with an even number of carbon atoms is metabolized to acetyl-CoA, which can enter the citric acid cycle. A fatty acid with an odd number of carbon atoms is metabolized to acetyl-CoA and...
-
The area of a rectangle is 30 cm 2 and its perimeter is 26 cm. Find the length and width of the rectangle.
-
The Apex Company sold a water softener to Marty Smith. The price of the unit was $350. Marty asked for a deferred payment plan, and a contract was written. Under the contract, the buyer could delay...
-
One thousand dollars is borrowed for one year at an interest rate of 1%per month. If the same sum of money could be borrowed for the same period at an interest rate of 12% per year, how much could be...
-
A sum of money Q will be received 6 years from now. At 5% annual interest the present worth now of Q is $60. At the same interest rate, what would be the value of Q in 10 years?
-
Prepare a 2017 T1 Return given the following information: Complete the T1 Income Tax Return and any relevant Schedules. Name: Mr. Henry Connor Age: 58 Years Old Province: Saskatchewan Gross Salary...
-
Date Aug 1 16 10 19 28 31 Activities Beginning inventory Purchase Sales Purchase Purchase Sales Units Acquired at Cost Units Sold at Retail 30 units @ $150 per unit 20 units @ $155 per unit 35 units...
-
Polaski Company manufactures and sells a single product called a Ret. Operating at capacity, the company can produce and sell 42,000 Rets per year. Costs associated with this level of production and...

Study smarter with the SolutionInn App


