The standard Gibbs energies of formation, f G, for KO 2 (s) and K 2 O(s)
Question:
The standard Gibbs energies of formation, ΔfG°, for KO2(s) and K2O(s) are -240.59 kJ mol‾1 and -322.09 kJ mol‾1, respectively, at 298 K. Calculate the equilibrium constant for the reaction below at 298 K. Is KO2(s) thermodynamically stable with respect to K2O(s) and O2(g) at 298 K?
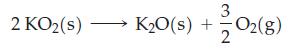
Transcribed Image Text:
2 KO₂ (s) 3 K₂O(s) + -O₂(g) 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
To calculate the equilibrium constant we can use the following equation G RT ln ...View the full answer

Answered By

BRIAN MUSINGA
I possess a Bachelors of Commerce degree(Marketing option) and am currently undertaking an MBA in marketing. I believe that I possess the required knowledge and skills to tutor in the subject named. I have also written numerous research academic papers much to the satisfaction of clients and my professors.
5.00+
2+ Reviews
17+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
All of the following are ways in which assets may be transferred so that they will not count as resources for Medicaid purposes except: Assets gifted into a trust are not subject to look-back if made...
-
The standard Gibbs energies of formation, f G, for Na 2 O(s) and Na 2 O 2 (s) are -379.09 kJ mol 1 and -449.63 kJ mol 1 , respectively, at 298 K. Calculate the equilibrium constant for the reaction...
-
At 298 K, the f G values for Li 2 O(s) and Li 2 O 2 (s) suggest that Li 2 O 2 (s) is thermodynamically more stable than Li 2 O(s). At 1000 K, however, the situation is reversed. The standard Gibbs...
-
A firm makes three types of furniture: beds, chairs and tables. The manufacturing times required to make them, the profit and capacity available at each work centre are given below; To make one unit...
-
A lawn care company started business on January 1, 2012. The company billed clients $105,000 for lawn care services completed in 2012. By December 31, the company had received $84,000 cash from...
-
Toys "R" Us, Inc. reported the following information (in U.S. millions) for the three fiscal years ended: Instructions (a) Calculate the gross profit margin and profit margin for Toys "R" Us for each...
-
8-4. What is the difference between the demographic and behavioral bases of market segmentation?
-
Sports Fanatic Company is a retail sporting goods store that uses accrual accounting for its records. Information on Sports Fanatic's operations are as follows: 1. The store has budgeted sales at...
-
Murphy owns 10% of an S corporation. He does not perform services for the company. Which statement is TRUE about Murphy? Murphy will pay self-employment tax on guaranteed payments and his reasonable...
-
The empirical formula of the mineral beryl is Be 3 Al 2 Si 6 O 18 . By using the several descriptions of silicate minerals just given as a guide, describe the structure of the silicate anion in beryl.
-
Consider the reaction Ca(OH) 2 (s) + Na 2 SO 4 (aq) CaSO 4 (s) + 2 NaOH(aq). (a) Write a net ionic equation for this reaction. (b) Will the reaction essentially go to completion? (c) What will be...
-
Use the following information to prepare a budgeted balance sheet for Marine Corporation at March 31. Show computations for the cash and owners equity amounts. a. March 31 inventory balance, $ 15,085...
-
Case # 4 Joseph Joseph, a 19-year-old African American college freshman. Yesterday he spent the afternoon drinking beer and taking shots of vodka with his fraternity brothers. After 6 glasses of beer...
-
1. Kaldor facts [50 points] Kaldor (1961) documented a set of stylized facts on the growth process of industrialized countries. We discussed these facts in lecture 2. Explain if and how the...
-
County has the Investment Activities recorded in its general fund: Tesla Stock: Cost $100, Fair Value on Jan 1x1: $200; Fair Value on Dec 31x2: $300 DJT Stock: Cost: $100; Fair Value on Jan 1x1:...
-
Pets World is a retailer of a popular blend of organic dog food produced by Natural Pets Company. On average, Pets World sells 600 cans per week. The wholesale price that Natural Pets Company charges...
-
Out Supply-Chaining the King of Supply Chainers, How easy (or hard) would it be for rivals like Walmart or Carrefour to adopt Tesco's data management techniques? (Please provide reference...
-
(a) Answer Exercise 4 under the additional requirement that the system includes a highway directly linking Evansville and Indianapolis. (b) If there must be a direct link between Fort Wayne and Gary...
-
Use the T account for Cash below to record the portion of each of the following transactions, if any that affect cash. How do these transactions affect the companys liquidity? Jan. 2 Provided...
-
Kiram Corporation purchased machinery on January 1, 2012, at a cost of $350,000. The estimated useful life of the machinery is 5 years, with an estimated salvage value at the end of that period of...
-
Paulson Corporation??s unadjusted trial balance at December 1, 2012, is presented below. The following transactions occurred during December.Dec. 2 Paulson purchased equipment for $16,000, plus sales...
-
Refer to the financial statements and the Notes to Consolidated Financial Statements of Tootsie Roll Industries in Appendix A. Instructions Answer the following questions. (a) What were the total...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


