The standard molar heats of combustion of C(graphite) and CO(g) are -393.5 and -283 kJ/mol, respectively. Use
Question:
The standard molar heats of combustion of C(graphite) and CO(g) are -393.5 and -283 kJ/mol, respectively. Use those data and that for the following reaction
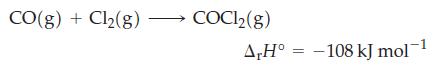
to calculate the standard molar enthalpy of formation of COCl2(g).
Transcribed Image Text:
CO(g) + Cl₂(g) COC1₂(g) A,H° -108 kJ mol-¹ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
Solution To calculate the standard molar enthalpy of formation of COCl2g we can u...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
When 1.000 g of gaseous butane, C4H10, is burned at 25oC and 1.00 atm pressure, H2O(l) and CO2(g) are formed with the evolution of 49.50 kJ of heat. a. Calculate the molar enthalpy of formation of...
-
Euler's original article about the Konigsberg Bridge Problem, which is dated 1736, presents a second similar problem with two islands, four rivers flowing around them, and 15 bridges connecting...
-
Zainab company has sold goods on credit RO 55,000 on 31st December 2020 and received RO 15,000 towards credit sales. The company had debit balance of RO 5500 and the balance in accounts receivable...
-
Retailers as well as manufacturers can apply just-in-time (JIT) to their inventory management. Both Best Buy and Circuit City want to know the impact of a JIT inventory system for their operating...
-
A marketing research firm is interested in determining whether there is a difference between the proportion of households in Chicago and the proportion of households in Milwaukee who purchase...
-
REPLACEMENT ANALYSIS The Dauten Toy Corporation currently uses an injection molding machine that was purchased 2 years ago. This machine is being depreciated on a straight-line basis, and it has 6...
-
Jim Haught, D.D.S., opened an incorporated dental practice on January 1, 2014. During the first month of operations, the following transactions occurred. Performed services for patients who had...
-
Gilder Corporation makes a product with the following standard costs: Standard Price or Rate Standard Cost Per Unit Direct materials Direct labor Variable overhead Standard Quantity or Hours 6.40...
-
a. Determine sample size: Determine sample size for each company using classical variables MPU estimation sampling. b. Analysis and evaluation: Assume the total audited value of the Company X sample...
-
Can a chemical compound have a standard enthalpy of formation of zero? If so, how likely is this to occur? Explain.
-
A 1.22 kg piece of iron at 126.5 C is dropped into 981 g water at 22.1 C. The temperature rises to 34.4 C. What will be the final temperature if this same piece of iron at 99.8 C is dropped into 325...
-
A classs instance variables must be declared outside of all _______ , and all instance variable declarations should be located at the _______ .
-
The purpose of this installment is to classify stock, bond, and mutual fund investments, explore tools for their evaluation and select these securities based on your investment philosophy and goals....
-
Jackson County Senior Services is a nonprofit organization devoted to providing essential services to seniors who live in their own homes within the Jackson County area. Three services are provided...
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
1. What gives stainless steels their good corrosion resistant properties? 2. Which stainless steel is the lowest cost and why? 3. What are some characteristics of Nickel Alloys? 4. What are the 2...
-
Problem 4. Determine the motion of a two-dimensional linear oscillator of potential energy V = kr
-
Why does drill feed increase with drill size?
-
State whether each statement is true or false. If false, give a reason. {purple, green, yellow} = {green, pink, yellow}
-
Morlan Corporation is preparing its December 31, 2010, financial statements. Two events that occurred between December 31, 2010, and March 10, 2011, when the statements were issued, are described...
-
Tina Bailey, a student of intermediate accounting, was heard to remark after a class discussion on segment reporting, All this is very confusing to me. First we are told that there is merit in...
-
Foley Corporation has seven industry segments with total revenues as follows. Penley $600 Cheng $ 225 Konami 650 Takuhi 200 KSC 250 Molina 700 Red Moon 275 Based only on the revenues test, which...
-
Assume today is Sept 1, 2021. Company IMineGold is a gold mining company producing gold with plans to increase its size and valuation over the next few years. The company has invested in developing...
-
Date Activities Units Acquired at Cost Units sold at Retail Jan. 1 Beginning inventory 235 units @ $ 16.00 = $ 3,760 Jan. 10 Sales 185 units @ $ 25.00 Jan. 20 Purchase 180 units @ $ 15.00 = 2,700...
-
Zo Sage opened a renovation business called Cranbrook Construction on August 3, 2020. During the first month of operations, the business completed the following transactions: (Click on the icon to...

Study smarter with the SolutionInn App


