The structure of the molecule allene, CH 2 CCH 2 , is shown here. Propose hybridization schemes
Question:
The structure of the molecule allene, CH2CCH2, is shown here. Propose hybridization schemes for the C atoms in this molecule.
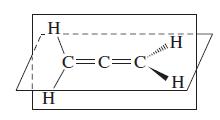
Transcribed Image Text:
H H C=C=C H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Based on the image you provided the hybridization schemes for the C atoms in allene are as fo...View the full answer

Answered By

William Otieno
I am a professional tutor and a writer with excellent skills that are important in serving the bloggers and other specialties that requires a great writer. The important aspects of being the best are that I have served so many clients with excellence
With excellent skills, I have acquired very many recommendations which have made it possible for me to survive as an excellent and cherished writer. Being an excellent content writer am also a reputable IT writer with essential skills that can make one turn papers into excellent result.
4.70+
83+ Reviews
354+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Indicate which of the following molecules and ions are linear, which are planar, and which are neither. Then propose hybridization schemes for the central atoms. (a) Cl 2 C = CCl 2 ; (b) N C C N;...
-
The Lewis structure for allene is Make a sketch of the structure of this molecule that is analogous to Figure 9.25. In addition, answer the following three questions: (a) Is the molecule planar? (b)...
-
Here are shown the atomic packing schemes for several different crystallographic directions for some hypothetical metal. For each direction the circles represent only those atoms contained within a...
-
A horizontal jet of water (at 10C) that is 6 cm in diameter and has a velocity of 20 m/s is deflected by the vane as shown. If the vane is moving at a rate of 7 m/s in the x-direction, what...
-
Georgia purchased an option on Greenacre from Pamela for $10,000. The option contract contained a provision by which Georgia promised not to assign the option contract without Pamelas permission....
-
What were the major innovations of the multi-divisional form?
-
LO3 For each of the following situations, determine the proper year for recognition of the income or deduction if the taxpayer is (1) a cash basis taxpayer and (2) an accrual basis taxpayer: a. Helen...
-
The trial balance of Yewlett Company includes the following balance sheet accounts, which may require adjustment. For each account that requires adjustment, indicate (a) The type of adjusting entry...
-
Learning Unit 5 Questions and Problems 1. Chapter 5. Objective Questions 3.4.5 and 6. II. A medical clinic is considering investing in a new diagnostic piece of equipment that will allow for a new...
-
The Nori & Leets Co. is one of the major producers of steel in its part of the world. It is located in the city of Steel-town and is the only large employer there. Steel-town has grown and prospered...
-
Angelic acid, shown below, occurs in sumbol root, a herb used as a stimulant. Represent the bonding in the angelic acid molecule by using the method in Figure 11-19 to indicate hybridization schemes...
-
Propose a bonding scheme that is consistent with the structure for propynal. 123 -C- -C 120 H -C- 1/7/7/196 120.4 pm 106 pm 146 pm 108 pm H 121 pm
-
Highlight the nature of political governance of sport and the implications for managers in the delivery of services and the implementation of policies.
-
Are some values in the class data grossly different from all the others? If so, check for errors in calculation or procedure that would allow to objectively eliminate the data. 2. Do the range values...
-
An aging analysis of Uli Limited's accounts receivable at December 3 1 , 2 0 2 4 and 2 0 2 3 , showed the following: Number of Days Outstanding Accounts Receivable Estimated Percentage Uncollectible...
-
(Linear momentum) Two jets of liquid, one with specific gravity 1.00 and the other with specific gravity 1.33, collide and form one homogeneous jet as shown in the figure below. Determine (a) the...
-
1. Define a person-centered model of care in LTC facilities. 2. Describe two leadership behaviors and two leadership qualities most conducive to moving long-term care organizations toward more...
-
question 5 all parts 8+0.5 = 4. Consider a system with a lead compensator Ge(s) = +0.13 followed by a plant G(s) = 10 Determine a value for a gain K on the error signal such that the phase margin...
-
Show the steps in the mechanism for eq. 9.18. OCH3 (9.18) CH,OH (excess) -H-H+(catalyst) OCH3
-
After Theorem 1.5 we note that multiplying a row by 0 is not allowed because that could change a solution set. Give an example of a system with solution set S0 where after multiplying a row by 0 the...
-
Operating Cycle what are some of the characteristics of a firm with a long operating cycle?
-
Cash Cycle what are some of the characteristics of a firm with a long cash cycle?
-
Sources and Uses for the year just ended, you have gathered the following information about the Holly Corporation. a. A $200 dividend was paid. b. Accounts payable increased by $500. c. Fixed asset...
-
In 1975 the price of a new house was $48,273. In 2020 the price of a new house is $185,524. How much has the price of housing increased over the entire time period in percentage terms? State your...
-
CHOP Inc., which makes only one product, Yester, has the following information available for the coming year. CHOP expects sales to be 27,000 units at $32 per unit. The current inventory of Yester is...
-
Huron Company produces a commercial cleaning compound known as Zoom. The direct materials and direct labor standards for one unit of Zoom are given below: Standard Quantity or Standard Hours Standard...

Study smarter with the SolutionInn App


