To what neutral atom do the following valence-shell configurations correspond? Indicate whether the configuration corresponds to the
Question:
To what neutral atom do the following valence-shell configurations correspond? Indicate whether the configuration corresponds to the ground state or an excited state.
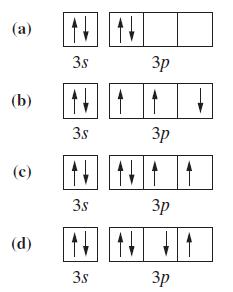
Transcribed Image Text:
(a) (b) (с) (d) 3.s 3s 3s N N 3.s 3р 3р 3p 3р
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
a Box a represents that this is in the excited state because the two electrons in the 2p are ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Does each of the configurations in Figure Q 41.6 represent a possible electron configuration of an element? If so, (i) identify the element and (ii) determine whether this is the ground state or an...
-
Does each diagram in Figure Q29.12 represent a possible electron configuration of a neutral element? If so, (i) Identify the element (ii) Determine if this is the ground state or an excited state. If...
-
(A) Which two of the following orbital diagrams are equivalent? (B) Does the following orbital diagram for a neutral species correspond to the ground state or an excited state? (c) 1s 2s A 1s 2s 2p...
-
Venator fund has a 5% front load. The fund had 13.1% return over the last 5 years. What is the actual annual return for investor invested in the fund for 5 years?
-
On the basis of Home Depot's response to environmentalist issues, describe the attributes (power, legitimacy, urgency) of this stakeholder. Assess the company's strategy and performance with...
-
If the Consumer Price Index rose from 109.6 to 133.8 over an 8 -year period, what was the equivalent compound annual inflation rate during the period?
-
A vendor has agreed to clean your hotel carpets at a very competitive price. In a telephone conversation with you, the vendor states that if it gets the contract, members of its staff will do your...
-
Methamphetamine (meth) is an addictive, synthetic drug made chiefly in small toxic labs (STLs) in homes, tents, barns, or hotel rooms. The manufacturing process is dangerous, often resulting in...
-
calculate the ecpected rate The table given below reports last five years data on annual rates of return (HPY) on two stocks Stock A 190 Stock B (96) -10 1 16 2 24 0 3 40 30 10 40 20 20 2 compute the...
-
All Mopped Up Company has journalized the adjusting entries for the period ending December 31, 2018, and posted the adjustments to the following T-accounts. (Click the icon to view the T-accounts.)...
-
What is the expected ground-state electron configuration for each of the following elements? (a) Mercury; (b) Calcium; (c) Polonium; (d) Tin; (e) Tantalum; (f) Iodine.
-
Which of the following electron configurations corresponds to the ground state and which to an excited state? (a) [B] (b) [C] (c) [N] (d) [O] 1s 25 N N 1s 25 2p 2p 1s 25 2p N N ^^ 1s 2s 2p
-
State and local governments use a large number of accounts in their balance sheets.
-
How has Biden Lowered premiums and out of pocket costs for millions of Americans?
-
Product costs using activity rates Body-Solid Inc. manufactures elliptical exercise machines and treadmills. The products are prouced in its Fabrication and Assembly production departments. In...
-
How to start an essay on the multigenerational workforce and your experiences working with each of the generations. Begin your essay with an introduction that outlines the current generations in the...
-
1. What does the phrase "cost of quality" mean? How might using this statement assist a company in addressing its quality issues? 2. What key distinctions exist between total quality human resource...
-
Does productivity in terms of output per labor our insure a company will be profitable? Why or why not? What questions should be asked to test whether productivity has increased? How do these answers...
-
Draw a compass rose and vector with magnitude v for each bearing. Find the angle made with the x-axis. a. 147 b. 204 c. 74 d. 314
-
Reread the discussion leading to the result given in (7). Does the matrix sI - A always have an inverse? Discuss.
-
Critique Fiedlers LPC theory. Are other elements of the situation important? Do you think Fiedlers assertion about the inflexibility of leader behavior makes sense? Why or why not?
-
Do you agree or disagree with Fiedlers assertion that leadership motivation is basically a personality trait? Why?
-
Compare and contrast the LPC and path-goal theories of leadership. What are the strengths and weaknesses of each?
-
Cullumber Tool Company's December 3 1 year - end financial statements contained the following errors. \ table [ [ , December 3 1 , 2 0 2 5 , December 3 1 , 2 0 2 6 ] , [ Ending inventory,$ 8 , 8 0 0...
-
which taxpayer will not file schedule c to report business activity ? A Bcky and Chris are married filing joint return they are the only owners of business and they materially participate The...
-
Below is the comparatve income statemont of C. Gabel, inc. Read the tocuirements. Requirement 1. Prepare a horitontal analytis of the comparative income stalement of C. Casbel. inc. Data table...

Study smarter with the SolutionInn App


