Together with the following data, to estimate the bond-dissociation energy of the F 2 molecule. Compare your
Question:
Together with the following data, to estimate the bond-dissociation energy of the F2 molecule.
![]()
Compare your result with the value listed in Table 10.3.
Table 10.3
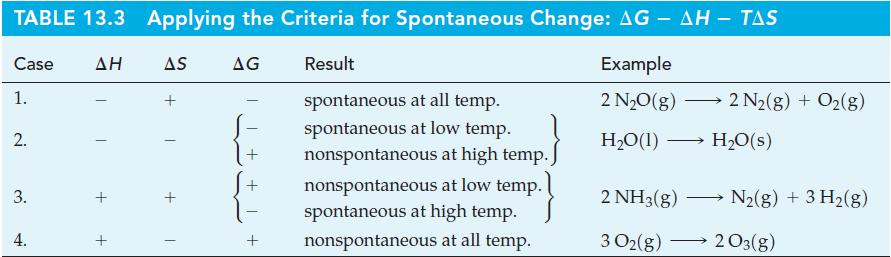
Transcribed Image Text:
F₂(g) - 2 F(g) A.G° = 123.9 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The image shows the following thermodynamic data for the dissociation of F2g into 2Fg G 1239 kJ mol ...View the full answer

Answered By

Mehwish Aziz
What I have learnt in my 8 years experience of tutoring is that you really need to have a friendly relationship with your students so they can come to you with their queries without any hesitation. I am quite hardworking and I have strong work ethics. Since I had never been one of those who always top in the class and always get A* no matter what, I can understand the fear of failure and can relate with my students at so many levels. I had always been one of those who had to work really hard to get decent grades. I am forever grateful to some of the amazing teachers that I have had who made learning one, and owing to whom I was able to get some extraordinary grades and get into one of the most prestigious universities of the country. Inspired by those same teachers, I am to be like one of them - who never gives up on her students and always believe in them!
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Consider the digital divide and how Smart Phones are increasing Internet access to the negative side of Smart Phones. Is there a balance between the advantages and disadvantages of this technology?...
-
Determine the sum or difference in clock 7 arithmetic. (4 - 5) - 6 9. 3
-
Estimate the cp of nitrogen at 300 kPa and 400 K, using (a) The relation in the above problem and (b) Its definition. Compare your results to the value listed in Table A2b.
-
Write the acceleration vector a at the point indicated as a sum of tangential and normal components. r(0) = (cos, sin 20), 0 =
-
Marie Corporation manufactures a fiber optic connector. The variable cost per unit is $16. The fixed cost per unit is $9. The companys desired ROI per unit is $3. Compute the markup percentage using...
-
What is the potential difference across a \(10 \mathrm{mH}\) inductor if the current through the inductor drops from \(150 \mathrm{~mA}\) to \(50 \mathrm{~mA}\) in \(10 \mu \mathrm{s}\) ?
-
How is it possible that a lifetime of experiences and accumulated knowledge can be stored in neurons? (190)
-
Briefly discuss the IASB/FASB convergence efforts in the area of postretirement-benefit accounting.
-
Which of the following statements regarding the selling price is in correct ? Group of answer choices: A lower selling price will decrease the contribution margin per unit. All of these statements...
-
Write an equation for the combustion of one mole of benzene, C 6 H 6 (l), and determine at 298 K if the products of the combustion are (a) CO 2 (g) and H 2 O(l), and (b) CO 2 (g) and H 2 O(g)....
-
The following r G values are given for 25 C. Combine the preceding equations, as necessary, to obtain r G values for the following reactions. Of reactions (a), (b), and (c), which is spontaneous in...
-
Following are selected transactions of Gamble Company: 1. Purchased 100 units of merchandise at $240 each: terms 2/10, n/30. 2. Paid the invoice in transaction I within the discount period. 3. Sold...
-
Construct a confidence interval for p-P2 at the given level of confidence. x =26, n =229, x2 = 31, n = 302, 95% confidence The researchers are % confident the difference between the two population...
-
AP 9-2 (Moving Expenses) In May of the current year, following a dispute with her immediate superior, Ms. Elaine Fox resigned from her job in Halifax and began to look for other employment. She was...
-
Minimize the number of states in the following DFA: A b b a a a b b b E B a a
-
You have two dashboards in the same workspace named Production and Manufacturing. Your company's Power BI administrator creates the following two dashboard data classifications: Medium Impact (MEDI)...
-
Question 2: Red Rocks Corporation was organized on September 1. Red Rocks encountered the following events during the first month of operations. a. Received $65,000 cash from the investors who...
-
A balanced delta-connected source has phase voltage Vab = 416 30 V and a positive phase sequence. If this is connected to a balanced delta-connected load, find the line and phase currents. Take the...
-
Refer to Example 9.15. Add the following functionality to this program: Allow the user to enter the cost of a gallon of gas on each trip and use a function, Cost() to calculate the cost of purchasing...
-
High-low, regression Pat Flip is the new manager of the materials storeroom for Serth manufacturing. Pat has been asked to estimate future monthly purchase costs for part #4599, used in two of Serths...
-
Learning curve, cumulative average-time learning model. Global Defense manufactures radar systems. It has just completed the manufacture of its first newly designed system, RS-32. Manufacturing data...
-
Learning curve, incremental unit-time learning model. Learning curve, incremental unit-time learning model, Assume the same information for Global Defense as in Exercise 10-29, except that Global...
-
Practical Corporation is liquidated, with Neha receiving property having an adjusted basis of $60,000 and an FMV of $100,000. The property is subject to a $75,000 mortgage, which Neha assumes. Neha's...
-
6. Last year Mason Inc had a total assets turnover of 1.33 and an equity multiplier of 1.75. Its sales were $195,000 and its net income was $10,549. The CFO believes that the company could have...
-
Cover-to-Cover Company is a manufacturer of shelving for books. The company has compiled the following cost data, and wants your help in determining the cost behavior. After reviewing the data,...

Study smarter with the SolutionInn App


