The following r G values are given for 25 C. Combine the preceding equations, as necessary,
Question:
The following ΔrG° values are given for 25 °C.
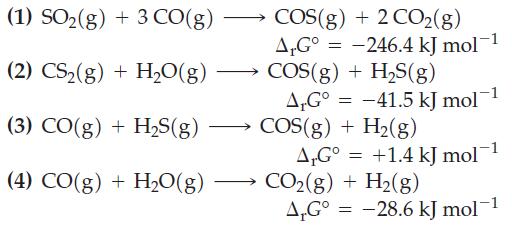
Combine the preceding equations, as necessary, to obtain ΔrG° values for the following reactions.
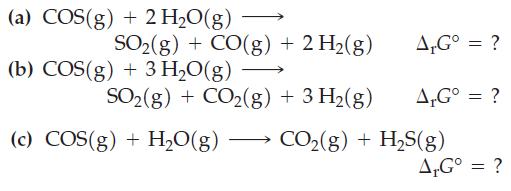
Of reactions (a), (b), and (c), which is spontaneous in the forward direction when reactants and products are present in their standard states?
Transcribed Image Text:
(1) SO₂(g) + 3 CO(g) (2) CS₂(g) +H₂O(g) (3) CO(g) + H₂S(g) (4) CO(g) + H₂O(g) COS(g) + 2 CO2(g) AG° = -246.4 kJ mol-1 COS(g) + H₂S(g) A,G° -41.5 kJ mol COS(g) + H₂(g) AG° +1.4 kJ mol-1 CO₂(g) + H₂(g) A.Gº = -28.6 kJ mol-¹ = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To obtain the ArG values for the given reactions you can sum the ArG values of the relevant reaction...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following r G values are given for 25 C. Combine the preceding equations, as necessary, to obtain r G values for each of the following reactions. Of reactions (a), (b), and (c), which would...
-
As the United States fell further and further into a recession in the years 2007-2009, the number of families who had to default on their mortgages and those who actually lost their homes grew....
-
A survey was conducted prior to the 2004 presidential election to explore the relationship between a persons religious fervor and their choice of a political candidate. Voters were asked how often...
-
Find an equation of the tangent plane at the given point. f(x, y) = x + y, (4,1)
-
What costs are excluded from the cost base when absorption-cost pricing is used to determine the markup percentage?
-
In some countries AC outlets near bathtubs are restricted to a Bi. 0 maximum of \(25 \mathrm{~V}\) to minimize the chance of dangerous shocks while bathing. A man is in the tub; the lower end of his...
-
How can the results of memory research be used to create more effective study techniques? (187)
-
Pedro Morales and Associates, a C.P.A. firm, uses job order costing to capture the costs of its audit jobs. There were no audit jobs in process at the beginning of November. Listed below are data...
-
Beginning Capital + Additional Investments +Revenues - Withdrawals - ______________ = Ending Capital
-
Together with the following data, to estimate the bond-dissociation energy of the F 2 molecule. Compare your result with the value listed in Table 10.3. Table 10.3 F(g) - 2 F(g) A.G = 123.9 kJ mol-1
-
At 298 K, for the reaction 2 PCl 3 (g) + O 2 (g) 2 POCl 3 (l), r H = -620.2 kJ mol -1 and the standard molar entropies, in J mol 1 K 1 , are PCl 3 (g), 311.8; O 2 (g), 205.1; and POCl 3 (l), 222.4....
-
Solve the given systems of equations by the method of elimination by addition or subtraction. 2x - 3y 3x + 2 = 2y 4 = 0
-
he previous three weeks of data is below for the sales of sheds at SHEDS INC. Calculate the forecast for the next perioud (week 4) using a two period weighted moving average using weights of 3 and 2....
-
/3 3) ST tan(x) - In(cosx) dx What is the value of u? us dulcis) What is the corresponding value of du? du= 1-5mx dx cosx You must show all of your work in the space below to earn full credit. 9/3 So...
-
Please use the file which provides the data to answer the problems 1-3. Problem 1) The time Students entered the classroom of OM 390, Introductory Operations Management, was recorded by the professor...
-
1) Factor the following Expressions (Write your factors only, don't show your work) a) 2x - 32 = c) 3x-2x-8= b) 2x-6x-8=
-
Bloomfield Inc. manufactures widgets. A major piece of equipment used to make the widget is nearing the end of its useful life. The company is trying to decide whether they should lease new equipment...
-
A three-phase balanced system with a line voltage of 202 V rms feeds a delta-connected load with Zp = 25 60. (a) Find the line current. (b) Determine the total power supplied to the load using two...
-
Write the statement to store the contents of the txtAge control in an Integer variable named intAge.
-
High-low method Ken Howard, financial analyst at JVR Corporation, is examining the behavior of quarterly maintenance costs for budgeting purposes. Howard collects the following data on machine-hours...
-
High-low method and regression analysis. Happy Business College has recently opened a restaurant as part of its hospitality major. For the first 10 weeks the manager did not estimate any costs, but...
-
High-low method, regression analysis. Anna Martinez, the financial manager at the Casa Real restaurant is checking to see if there is any relationship between newspaper advertising and sales revenues...
-
Winter Time Adventures is going to annual dividend if $2.61 a share on its common stock next week. This year, the company paid a dividend of $2.50 a share. The company adheres to a constant rate of...
-
Small Factory : -Regular time 8 hours per day. -1 hour daily lunch break. -25 working days per month. -50 workers. -Worker productivity 2.5 units per hour. -sold for $ 150 per unit. -cost of Labor...
-
$500 is invested for 7 years at 10 % p.a. simple interest. How much will the investment be worth after this period

Study smarter with the SolutionInn App


