Toluene-2,4-diisocyanate is used in the manufacture of polyurethane foam. An incomplete structure is shown below. Describe the
Question:
Toluene-2,4-diisocyanate is used in the manufacture of polyurethane foam. An incomplete structure is shown below. Describe the hybridization scheme for the atoms marked with an asterisk, and indicate the values of the bond angles marked α and β.
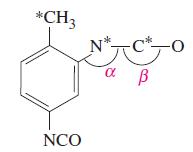
Transcribed Image Text:
*CH3 NCO *C -0 a В N*-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
Hybridization Scheme for Atoms Marked with an Asterisk The carbon atom marked with an asterisk is sp...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The head box process is used in the manufacture of paper to transform the pulp slurry flow into a jet of 2 cm and then spread it onto a mesh belt [22]. To achieve desirable paper quality, the pulp...
-
The horizontal range and the maximum height of a projectile are equal. The angle of projection of the projectiles is: (a) 0 = tan() (b) 0 = tan (4) (c) 0 = tan (2) (d) 0 = 45
-
Two non-mixing liquids of densities p and np (n> 1) are put in a container. The height of each liquid is h. A solid cylinder of length L and density d is put in this container. The cylinder floats...
-
Samson Tile produces its product in two processing departments: Forming and Finishing. The following T-account shows the Forming Department's Work in Process Inventory at August 31 prior to...
-
An artist once produced a painting now called The Plains of Meudon. For a while, the parties in this case thought that the artist was Theodore Rousseau, a prominent member of the Barbizon school, and...
-
Design appropriate OP AMP circuits that will realize each of the functions in Problem 6-23. The OP AMPs available have a maximum \(K\) of 10,000 and a \(V_{\mathrm{CC}}= \pm 15 \mathrm{~V}\). Data...
-
LO5 What is the statute of limitations, and what role does it play in the filing of tax returns?
-
Crimson Tide Music Academy offers lessons in playing a wide range of musical instruments. The unadjusted trial balance as of December 31, 2021, appears below. December 31 is the company?s fiscal...
-
Profits have been decreasing for several years at Pegasus Airlines. In an effort to improve the companys performance, the company is thinking about dropping several flights that appear to be...
-
WAR (We Are Rich) has been in business since 1985. WAR is an accrual method sole proprietorship that deals in the manufacturing and wholesaling of various types of golf equipment. Hack & Hack CPAs...
-
The anion I 4 2- is linear, and the anion I 5 - is V-shaped, with a 95 angle between the two arms of the V. For the central atoms in these ions, propose hybridization schemes that are consistent with...
-
As discussed in Are You Wondering 11-1, the sp hybrid orbitals are algebraic combinations of the s and p orbitals. The required combinations of 2s and 2p orbitals are (a) By combining the appropriate...
-
Make a sketch of a concentration cell employing two Zn/Zn 2+ halfcells. The concentration of Zn 2+ in one of the half-cells is 2.0 M, and the concentration in the other half-cell is 1.0 * 10 -3 M....
-
Mr. A and B agreed to start a business agreed to share profit and loss based the condition that will profit only when there is profit in excess of BD 10,000 this from of business is called as:...
-
L= {a'e"b"d' | i=1+m and l,m,n 20] a. Write at least 10 strings of the above language in increasing order of string length. b. Write Context Free Grammar (CFG) for the above language.
-
The Beta Co. shows the following results of operation on Dec. 31. Variable cost Fixed costs Direct materials P512,500 Direct labor 575,000 Manufacturing overhead 400,000 P212,500 For the year then...
-
Explain in details the reasons for your classifications. Classify the following processes as batch, continuous, or semibatch, and transient or steady- state. 1. A balloon is filled with air at a...
-
Question 5. A first responder drone of mass m slug is launched with a velocity vo ft/sec and constant engine force F from a level ground and moves vertically upward to discover a sense of life in a...
-
Give the structure of each product. LI iAlli, o exCeSS H Ni, heat a. CH3C b. CH- CH-CH-O CH-CH- CH3 1. NaBH 2. H2O, H 10% Jones reagent C. d. Ag,O e. CH,CH=CHCHO
-
Abandonment Decisions Allied Products, Inc., is considering a new product launch. The firm expects to have annual operating cash flow of $25 million for the next 10 years. Allied Products uses a...
-
Pricing Convertibles you have been hired to value a new 25-year callable, convertible bond. The bond has a 7.20 percent coupon, payable annually. The conversion price is $1.60, and the stock...
-
WACC on the most basic level, if a firms WACC is 12 percent, what does this mean?
-
which of the following stateents is consistent with the balance sheet model of a firm a. longterm investment decision b. shareholder value equals long term liabilities minus short term liabilities c....
-
please show all methods for these please pleaseee! y 4. Equity at start of year 120,000 Sales revenue 175,000 Current liabilities at end of the year 90,000 Non-current liabilities at end of the year...
-
if the returns between two assets are negatively correlated, then the standard deviation of a portfolio made up of the two assets is: A) equal to a weighted average of the individual asset's standard...

Study smarter with the SolutionInn App


