Two of the substances listed here are highly soluble in water, two are only slightly soluble in
Question:
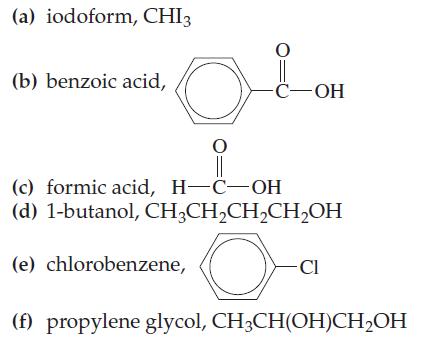
Two of the substances listed here are highly soluble in water, two are only slightly soluble in water, and two are insoluble in water. Indicate the situation you expect for each one.
Transcribed Image Text:
(a) iodoform, CHI3 (b) benzoic acid, O O || (c) formic acid, H-C-OH (d) 1-butanol, CH₂CH₂CH₂CH₂OH O (e) chlorobenzene, -C-OH - CI (f) propylene glycol, CH3CH(OH)CH₂OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Substance Solubility Explanation iodoform CHI3 Highly soluble Iodoform is a nonpolar molecule but it ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Benzoic acid, C 6 H 5 COOH, is much more soluble in NaOH(aq) than it is in pure water. Can you suggest a reason for this? The structural formula for benzoic acid is given in Exercise 5(b). Exercise...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
A sealed flask contains water and oxygen gas at 25C. The O 2 gas has a partial pressure of 1.5 atm. (a) What is the concentration of O 2 in the water? (b) If the pressure of O 2 in the flask is...
-
Green Pastures is a 400-acre farm on the outskirts of the Kentucky Bluegrass, specializing in the boarding of broodmares and their foals. A recent economic downturn in the thoroughbred industry has...
-
Plaintiff visited South Chicago on January 10, 2008, seeking a new 2008 Nissan Versa (Versa) with manual transmission, anti-lock brakes, and other features. He was told by the employees of South...
-
Do you consider companies make full use of situational audits and environmental analysis in their decision-making?
-
During the busiest season of the year, Green-Gro Fertilizer produces two types of fertilizers. The standard type (X) is just fertilizer, and the other type (Y) is a special fertilizer and weed-killer...
-
! Required information [ The following information applies to the questions displayed below. ] The management of Niagara National Bank is considering an investment in automatic teller machines. The...
-
At 0 C and an O 2 pressure of 1.00 atm, the aqueous solubility of O 2 (g) is 48.9 mL O 2 per liter. What is the molarity of O 2 in a saturated water solution when the O 2 is under its normal partial...
-
Describe a case in which the liquid and vapor curves in a diagram such as Figure 14-16 would converge into a single curve. Is such a case likely to exist? Figure 14-16 Pressure, mmHg 100.0 90.0 80.0...
-
What taxes make up Payroll Tax Expense? LO.1
-
A. For a certain two-dimensional, incompressible flow field the velocity component in the y direction is given by v = 3xy + xy 1. (05 pts) Short answer, what is the condition for this flow field to...
-
Cho0se a hazardous material to cr3ate a pr3sentation on (i.e. sulfuric acid, explosives, used needles, there are many types of hazardous materials) Cr3ate a presentation (P0werPoint, Open0ffice...
-
If det [a b] = c d 2 -2 0 a. det c+1 -1 2a d-2 2 2b -2 calculate:
-
! Required information [The following information applies to the questions displayed below.] Littleton Books has the following transactions during May. May 2 Purchases books on account from Readers...
-
4) Consider the table to the right that shows the number of free samples () and number of protein shakes sold (y). a)Complete the table [3 marks] b) Find the equation of the line of best fit X x y 8...
-
Construct the Bode magnitude and phase plots for H(s) = 40(s + 1) / (s + 2) (s + 10), s = j
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
In each case, state which of the two securities is likely to provide the higher return: (a) When the stock price rises (stock or convertible bond?). (b) When interest rates fall (straight bond or...
-
In 1996 Marriott International made an issue of LYONS. The bond matured in 2011, had a zero coupon, and was issued at $532.15. It could be converted into 8.76 shares. Beginning in 1999 the bonds...
-
The companys decision to issue warrants should depend on the managements forecast of likely returns on the stock. Do you agree?
-
The price of a vacation home is currently $314,041. If the price of vacation homes is increasing at a rate of 3.45% per year, how much would a vacation home cost in 9 years?
-
1a) AA Corporation's stock has a beta of 0.8. The risk-free rate is 4%, and the expected return on the market is 12%. What is the required rate of return on AA's stock? Do not round intermediate...
-
Your are required to present one or two audit issue (s) based on the topic of *Important issues in financial statement audit*. 1. The primary goal of your audit issue(s) is not to judge whether the...

Study smarter with the SolutionInn App


