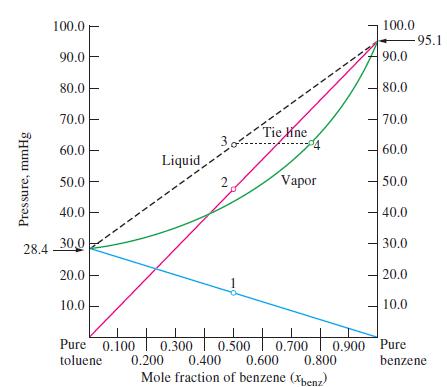
Describe a case in which the liquid and vapor curves in a diagram such as Figure 14-16
Question:
Describe a case in which the liquid and vapor curves in a diagram such as Figure 14-16 would converge into a single curve. Is such a case likely to exist?
Figure 14-16
Transcribed Image Text:
Pressure, mmHg 100.0 90.0 80.0 70.0 60.0 50.0 40.0 28.4 30,0 20.0 10.0 Liquid Pure 0.100 toluene 2 1 Tie ne Vapor 100.0 90.0 80.0 70.0 60.0 50.0 40.0 30.0 20.0 -95.1 10.0 + to + 0.300 0.500 0.700 0.900 Pure 0.400 0.200 0.600 0.800 benzene Mole fraction of benzene (Xbenz)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The liquid and vapor curves in a diagram such as Figure 1416 would converge into a single curve at t...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
We noted in Figure 14-17 that the liquid and vapor curves taken together outline a lens-shaped region when the normal boiling points of benzene-toluene solutions are plotted as a function of mole...
-
A constant-pressure R-134a vapor separation unit separates the liquid and vapor portions of a saturated mixture into two separate outlet streams. Determine the flow power needed to pass 6 L/s of...
-
A methanolwater feed stream is introduced to a vaporizer in which a molar fraction f of the feed is vaporized. The feed has a methanol mole fraction of x F = 0.4, and the vaporizer operates at a...
-
In the financial market, what causes a movement along the demand curve? What causes a shift in the demand curve?
-
The Current Designs staff has prepared the annual manufacturing budget for the rotomolded line based on an estimated annual production of 4,000 kayaks during 2013. Each kayak will require 54 pounds...
-
Johanna Marra and Eric Nazzaro began a romantic relationship in October 2013. That previous July, Nazzarro had purchased a duplex that he intended to renovate. Nazzarro rented out the top floor while...
-
Undertake PEST analyses of the external environment
-
1. What evidence is there in this case that BP simply addresses fines as a cost of doing business? 2. BP chief executive Tony Hayward argued that changing the culture of a 100,000 person company...
-
P7-5 (Algo) Evaluating the LIFO and FIFO Choice When Costs Are Rising and Falling LO7-2, 7-3 [The following information applies to the questions displayed below.] Income is to be evaluated under four...
-
Two of the substances listed here are highly soluble in water, two are only slightly soluble in water, and two are insoluble in water. Indicate the situation you expect for each one. (a) iodoform,...
-
A solution is prepared by dissolving 95 g NH 4 Cl in 200.0 g H 2 O at 60 C. (a) What mass of NH 4 Cl will recrystallize when the solution is cooled to 20 C? (b) How might we improve the yield of NH 4...
-
02 What is a communications protocol?
-
Determine the mean number of credit cards based on the raw data. (b) Determine the standard deviation number of credit cards based on the raw data. (c) Determine a probability distribution for the...
-
B Harry is a county Department of Social Services worker whose clients consist primarily of poor, female-headed families receiving public assistance. During one of his meetings with Dora, a single...
-
1 A, Weakly coupled carts (20 points) m m2 Figure 1: A system of two masses and three springs. A symmetric two degree of freedom system consists of two identical rigid masses m = m = m pictured in...
-
A farmer has an acre of specialty vegetables and is preparing for the summer harvest. Historically, this acre has yielded an average of 2,100 lbs of product with a standard deviation of 950 lbs. A...
-
Solve 3x 82+22 = (4).
-
Draw the Bode plots for H() = 50(j + 1) / j(-2 + 10 j + 25)
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
Financing with convertible debt is especially appropriate for small, rapidly growing, or risky companies. Explain why.
-
The Pork Barrel Company has issued three-year warrants to buy 12 percent perpetual debentures at a price of 120 percent. The current interest rate is 12 percent and the standard deviation of returns...
-
The B.J. Services warrant is described in Section 23.1. How would you use the BlackScholes formula to compute the value of the warrant immediately after its issue, assuming a stock price of $19 and a...
-
For the buyer of 72 Jul Call, calculate the profit or loss (per contract) on the expiration date, when the spot price for CD is $0.7350
-
What if Timcos dividends grow at 6% for one year, than at 10% forever after that?
-
23. The employer's portion of payroll taxes amounted to $1,000 for social security, $400 for state unemployment, $200 for federal unemployment, and $400 for worker's compensation. Record the journal...

Study smarter with the SolutionInn App


