Use Coulombs law to verify the conclusion concerning the relative strengths of the attractive forces in the
Question:
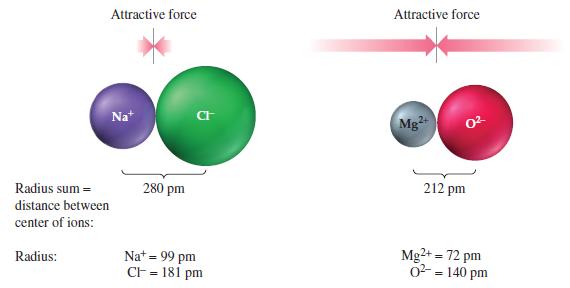
Use Coulomb’s law to verify the conclusion concerning the relative strengths of the attractive forces in the ion pairs Na+Cl- and Mg2+O2- presented in Figure 12-36.
Figure 12-36
Transcribed Image Text:
Attractive force Radius sum= distance between center of ions: Radius: Nat 280 pm CI Na+ = 99 pm CF = 181 pm Attractive force Mg²+ 0²- 212 pm Mg2+ = 72 pm 0² = 140 pm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To verify the conclusion concerning the relative strengths of the attractive forces in the ion pairs NaCl and Mg2O2 presented in Figure 1236 using Cou...View the full answer

Answered By

Wonder Dzidzormenu
As a professional accountant and a teacher, I explain account ing concepts in a more practical way that makes students more connected to the subject.
With over 10 years of teaching accounting , I offer a well constructed , easily understood and in-depth explanations to students questions.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family. The Incisors own a rental beach house in Hawaii. The beach house was rented for the full year during 2012...
-
Use Lenzs law to answer the following questions concerning the direction of induced currents. (a) What is the direction of the induced current in resistor R in Figure P31.28a when the bar magnet is...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
Listed below are measured amounts of caffeine (mg per 12 oz of drink) obtained in one can from each of 20 brands (7-UP, A&W Root Beer, Cherry Coke, . . . , Tab). Are the statistics representative of...
-
James sells and delivers to Gerald on June 1 certain goods and receives from Gerald at the time of delivery Geralds check in the amount of $9,000 for the goods. The following day, Gerald is...
-
A large elevated tank (Tank 1) is being used to supply water at 60F to two tanks at a lower elevation (Tanks 2 and 3) as shown in Figure P4.28. All pipes are 5-nom sch 40 commercial steel. The...
-
How does Mindfulness connect with the concept of authentic leadership?
-
John Weiss recently started a lawn care service named The Grass Is Always Greener, Inc. The following transactions occurred during the companys first month of business. Enter each of the following...
-
Question 1 (15 Marks) Part A (7 Marks) On 1 July 2021, Beta Ltd entered into a lease agreement to lease a machine. The lease gave Beta Ltd the sole right to use of the machine and receive all the...
-
Honolulu Shirt Shop has very seasonal sales. Assume that for next year management is trying to decide whether to establish a sales budget based on average sales or on sales estimated by quarter. The...
-
Will the mineral villaumite (NaF) or periclase (MgO) have a higher Mohs hardness value (see Exercise 67)? Exercise 67 The hardness of crystals is rated based on Mohs hardness values. The higher the...
-
Are the fullerenes network covalent solids? What makes them different from diamond and graphite? It has been shown that carbon can form chains in which every other carbon atom is bonded to the next...
-
The number (in thousands) of new, privately owned housing units completed in the United States is shown in the table. Here x represents the number of years since 2006, and y represents total housing...
-
You've decided to build a radio to listen to your favourite FM radio station, which broadcasts at 101.5 MHz. For the tuner, you'll be using an RLC circuit, but the only inductor you happen to have on...
-
You are the lead buyer for a large healthcare organization in British Columbia and have been tasked with leading the procurement of a new CAT Scan Machine. Outline four steps to prepare and call a...
-
A circuit is composed of a coil having N turns and area A. Its leads are connected to a combination of resistors, as shown in (Figure 1). All three resistors have the same resistance R. The coil is...
-
Let two planes be given by 2x-y+z = 8 and z = x+y-5 (a) Find the angle between the two planes. Leave your answer in degrees and round to the nearest tenth. (b) Find the vector equation of the line of...
-
9-2. The profile of a gear tooth shown in Fig. P9.2 is approximated by the trigonometric equation y(x) = a. Estimate the area A using eight rectangles of equal width A x = 1/8, b. Calculate the exact...
-
Show how diazonium ions could be used to synthesize a. p-chlorobenzoic acid from p-chloroaniline b. m-iodochlorobenzene from benzene c. m-iodoacetophenone from benzene d....
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
Calculating EAR a local finance company quotes a 15 percent interest rate on one-year loans. So, if you borrow $20.000, the interest for the year will be $3.000. Because you must repay a total of...
-
Calculating Present Values a 5-year annuity often $6,000 semiannual payments will begin 9 years from now, with the first payment coming 9.5 years from now. If the discount rate is 10 percent...
-
Calculating Annuities Due As discussed in the text, an ordinary annuity assumes equal payments at the end of each period over the life of the annuity. An annuity due is the same thing except the...
-
Derek plans to retire on his 65th birthday. However, he plans to work part-time until he turns 71.00. During these years of part-time work, he will neither make deposits to nor take withdrawals from...
-
Penske Ltd has a standard deviation of returns of 18% and a correlation with the market portfolio of 0.8. The market portfolios expected return is 14%, its standard deviation of returns is 12%, and...
-
What is the quoted price of a bond maturing in 12 years with a coupon rate of 9 percent, paid semiannually, that has a YTM of 13 percent? (Please round to the nearest hundredth)

Study smarter with the SolutionInn App


