Use data from Figure 9-1 and equation (9.1) to estimate the density of the recently discovered element
Question:
Use data from Figure 9-1 and equation (9.1) to estimate the density of the recently discovered element 114.
Figure 9-1
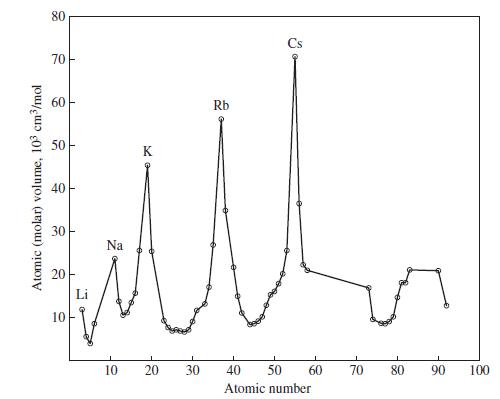
Eq. 9.1
![]()
Transcribed Image Text:
Atomic (molar) volume, 103 cm³/mol 80 70 60 50 40 30 20 10 T Na 10 K 20 30 Rb Cs 40 50 Atomic number 60 70 80 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
To estimate the density of element 114 using the data from Figure 91 and equation 91 ...View the full answer

Answered By

David Muchemi
I am a professional academic writer with considerable experience in writing business and economic related papers. I have been writing for my clients who reach out to me personally after being recommended to me by satisfied clients.
I have the English language prowess, no grammatical and spelling errors can be found in my work. I double-check for such mistakes before submitting my papers.
I deliver finished work within the stipulated time and without fail. I am a good researcher on any topic especially those perceived to be tough.
I am ready to work on your papers and ensure you receive the highest quality you are looking for. Please hire me to offer my readily available quality service.
Best regards,
4.60+
27+ Reviews
61+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Buoyancy correction factor: mtrue/mread = 1.000 3244] [Interpolated density of water at 23.3 C: 0.997 468 9 g/mL] [HCl concentration at 20 C: 0.102343 M] Reagents: The molar mass and density of...
-
Estimate the density of the water 6.0 km deep in the sea. (Se Table9-1 and Section 9-5 regarding bulk modulus.) By what fraction does it differ from the density at the surface?
-
Use the Arrhenius equation (Section 2-1) to estimate the ratio of the rate constants k for the reactions of a C-H bond in methane with a chlorine atom and with a bromine atom at 25C. Assume that the...
-
(a) By what percentage does your rest mass increase when you climb 30 m to the top of a ten-story building? Are you aware of this increase? Explain. (b) By how many grams does the mass of a 120-g...
-
Refer to the data in Exercise 6-31. Compute the predetermined overhead rate assuming that Tiger Furnishings uses machine-hours to allocate overhead costs.
-
Let f (x) = 2x cos(2x) (x 2)2 and x0 = 0. a. Find the third Taylor polynomial P3(x), and use it to approximate f (0.4). b. Use the error formula in Taylor's Theorem to find an upper bound for the...
-
Burke Copy Center purchased a machine on January 1, 1991, for $180,000 and estimated its useful life and salvage value at ten years and $30,000, respectively. On January 1, 1996, the company added...
-
The Inder Corporation is experiencing a temporary cash shortage and decides to factor a group of its accounts receivable. The factor accepts $80,000 of Inders accounts receivable, remits 90% of the...
-
FDNACCT has the following information on October 31, 2021. Assets P213,000 Liabilities 85,000 Capital, beginning 98,000 Revenues 115,200 Expenses ? Drawings None How much is the total expenses of the...
-
A drunk starts out from a lamppost in the middle of a street, taking steps of equal length either to the right or to the left with equal probability. What is the probability that the man will again...
-
Refer only to the periodic table on the inside front cover, and determine which is the largest atom: Sc, Ba, or Se.
-
When the ionization energies of a series of isoelectronic atoms and ions are compared, an interesting relationship is observed for some of them. In particular, if the square root of the ionization...
-
For each of the following independent cases, compute cash flows from operating activities. Assume the list below includes all balance sheet accounts related to operating activities. (Amounts in...
-
Decided to embark on a personal improvement project centered around time management after reviewing the insightful workbook by Neuhauser et al. (2004). My decision was influenced by my recognition...
-
You are the Senior Manager of IAuditYou LLP, you were recently assigned to take over a very important client for the company, The engagement partner, Max Roff, has been the audit partner for the past...
-
To create 3 scenarios (positive, neutral and negative) for the development of the restaurant. Include the following important factors in your assessments: border trade as one of the most important...
-
Description of market segment Aged between 34 and 58 Regular commuters Clerical or professional Income over $50K Moderately price-sensitive but may see higher price as an indicator of quality...
-
How has the job of the manager changed over time (since the publication of the Mintzberg article below)? What factors have contributed to shifts in the manager's role? What new roles are managers...
-
In the Western blot shown here, proteins were isolated from red blood cells and muscle cells from two different individuals. One individual was unaffected, and the other individual suffered from a...
-
What is the purpose of the journal wizard?
-
How does conformance quality differ from design quality? Explain.
-
Name two items classified as prevention costs.
-
Distinguish between internal failure costs and external failure costs.
-
The Gilmore Insurance Group earned a profit of $10,000 in its first month of business. Then, its profit increased by 3% each month for the next two years. What is the total profit that the Gilmore...
-
BluStar Company has two service departments, Administration and Accounting, and two operating departments, Domestic and International. Administration costs are allocated on the basis of employees,...
-
33 34 On January 1, 2020, Samford Company collected $6,000 in advance from a customer for services to be provided evenly over the next six months. If Samford prepares adjustments on January 31, the...

Study smarter with the SolutionInn App


