Use data from Figure 9-11 to predict the type of cubic unit cell adopted by (a) CaO;
Question:
Use data from Figure 9-11 to predict the type of cubic unit cell adopted by
(a) CaO;
(b) CuCl;
(c) LiO2 (the radius of the O2- ion is 128 pm).
Figure 9-11
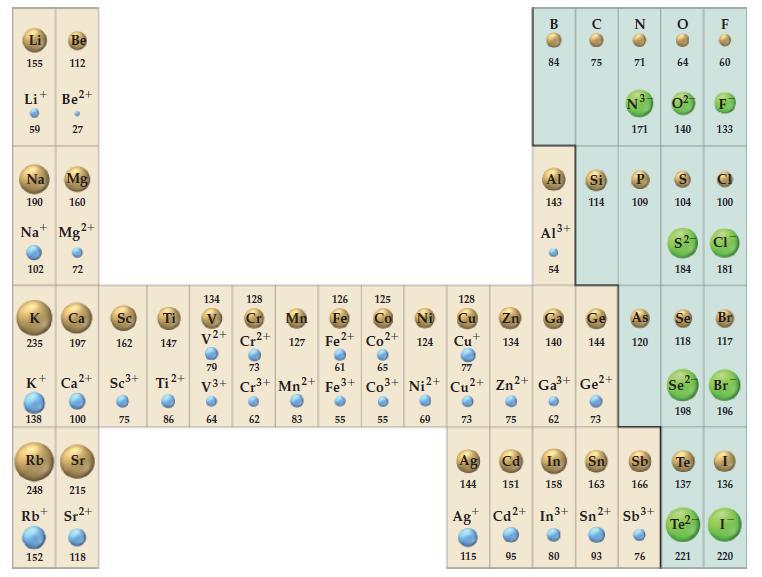
Transcribed Image Text:
155 59 Li+ Be²+ 102 K Na Mg 190 160 Na Mg2+ 235 Be 112 138 27 152 72 K+ Ca²+ Sc³+ Ca Sc Ti 197 162 147 100 Rb 248 215 Rb Sr²+ Sr 118 75 Ti ²+ 86 134 128 V2+ 79 V3+ 64 ២ Cr2+ 73 Cr³+ 62 126 Mn Fe 127 Fe2+ 2+ Mn2 83 61 Fe 3+ 55 125 Co2+ 65 Co3+ 55 Ni 124 Ni²+ 69 73 Ag 144 128 Cu Zn Ga Cu 134 140 с 115 75 B Cd 151 84 Al 143 A1³+ 95 54 77 Cu²+ Zn²+ Ga³+ Ge²+ 3+ 62 CO с 80 75 Ge 144 ● Si P 114 109 73 N ZO 93 71 N3 0² F 171 140 133 As 120 64 76 104 $2 In Sn Sb Te 158 163 166 137 + Ag Cd2+ In3+ Sn²+ Sb³+ 184 Se 118 Se 198 Te²- 60 221 100 CI 181 Br 117 Br 196 136 220
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To predict the type of cubic unit cell adopted by a compound we need to compare the radii ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Figure 9-9 to predict the type of cubic unit cell adopted by (a) BaO; (b) CuI; (c) LiS 2 . (The radii of Ba 2+ and S 2 - ions are 135 and 198 pm, respectively.) Figure 9-9 Tne/ao 9 8 7...
-
The table below lists the ionic radii for the cations and anions in three different ionic compounds. Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of...
-
Mn crystallizes in the same type of cubic unit cell as Cu. Assuming that the radius of Mn is 5.6% larger than the radius of Cu and the density of Cu is 8.96 g/cm3, calculate the density of Mn.
-
Prerequisite: You will be using "utils.py" from Python 2 Assignment Task 1) In "utils.py," add a Python function called "calculate_fourier_coefficients" that calculates the coefficients of a Fourier...
-
Bishop Logging Company is a large, family-owned logging contractor formed in the low country of South Carolina. Bishop Logging has traditionally harvested pine timber. However, Bishop Logging began...
-
In a debate, Eds best criticism of utilitarianism is that it a. encourages unethical behavior. b. fosters conformance with societys standards. c. mandates acting in an employers best interests. d....
-
What is most important to you? To your organization? How can you ensure that the values you and others hold are mutually discussed and fully understood? How can you live your most deeply held values?
-
Firenza Company manufactures specialty tools to customer order. Budgeted overhead for the coming year is: Purchasing ...... $40,000 Setups .......... 37,500 Engineering ...... 45,000 Other .............
-
Shortening the credit period A firm is contemplating shortening its credit period from 40 to 30 days and believes that, as a result of this change, its average collection period will decline from 47...
-
You are the audit senior on the audit of Great Eastern Hotel (GEH). This two-star hotel is located in a major coastal city and as such is prone to seasonal fluctuations. The 200-room hotel is open...
-
Potassium chloride has the same crystal structure as NaCl. Careful measurement of the internuclear distance between K + and Cl - ions gave a value of 314.54 pm. The density of KCl is 1.9893 g/cm 3 ....
-
Two views, a top and side view, for the unit cell for rutile (TiO 2 ) are shown here. (a) How many titanium atoms (blue) are in this unit cell? (b) How many oxygen atoms (red) are in this unit cell?
-
Given the project shown in Figure of Chapter 5, assume that a facility used by activities c and d is scarce. To which activity would you assign the facility first, given the following rules? (a)...
-
What are the elements of partnership ?
-
1. (2x+3)dx
-
Evaluate the following definite integrals as limit of sums: 1. (x (x-x)dx [(2x 2. (2x+5x)dx 3. (2x + +3x+1)dx
-
Evaluate as limit of sums Jr- 3+1dx 1
-
Naphthalene is colorless, but its isomer azulene is blue. Which compound has the lower-energy pi electronic transition? naphthalene azulene
-
How do individual companies respond to economic forces throughout the globe? One way to explore this is to see how well rates of return for stock of individual companies can be explained by stock...
-
Calculating Annuity Values Dinero Bank offers you a $30,000, seven-year term loan at 8 percent annual interest. What will your annual loan payment be?
-
Calculating Perpetuity Values The Maybe Pay Life Insurance Co. is trying to sell you an investment policy that will pay you and your heirs $20,000 per year forever. If the required return on this...
-
Calculating Perpetuity Values In the previous problem, suppose a sales associate told you the policy costs $280,000. At what interest rate would this be a fair deal?
-
You have been hired by Internal Business Machines Corporation (IBM) in their capital budgeting division. Your first assignment is to determine the free cash flows and NPV of a proposed new type of...
-
Burger King recently launched Real Meals in select markets to deliver an important message about mental health. Real Meals come in five varieties, including a Pissed Meal (for when youre mad) and a...
-
If banks suffer loan losses in excess of their loan-loss-reserves, their Capital Adequacy is unaffected. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an...

Study smarter with the SolutionInn App


