Use data from Figure 9-9 to predict the type of cubic unit cell adopted by (a) BaO;
Question:
Use data from Figure 9-9 to predict the type of cubic unit cell adopted by
(a) BaO;
(b) CuI;
(c) LiS2.
(The radii of Ba2+ and S2- ions are 135 and 198 pm, respectively.)
Figure 9-9
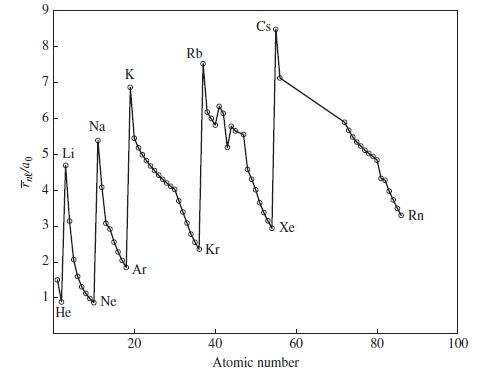
Transcribed Image Text:
Tne/ao 9 8 7 6 5 Li 3 2 1 He Na Ne K Ar 20 Rb Kr Csa Xe 40 60 Atomic number 80 Rn 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
To predict the type of cubic unit cell adopted by a compound we can use the following guidelines Sim...View the full answer

Answered By

Sagar Kumar
I am Mechanical Engineer with CGPA of 3.98 out of 4.00 from Pakistan. I went to Government Boys Degree College, Sehwan for high school studies.
I appeared in NUST Entrance Exam for admission in university and ranked #516. My mathematics are excellent and I have participated in many math competitions and also won many of them. Recently, I participated in International Youth Math Challenge and was awarded with Gold Honor. Now, I am also an ambassador at International Youth Math Challenge,
I have been teaching when I was in 9th class class year 2012. I have taught students from 6th class to university level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Figure 9-11 to predict the type of cubic unit cell adopted by (a) CaO; (b) CuCl; (c) LiO 2 (the radius of the O 2 - ion is 128 pm). Figure 9-11 155 59 Li+ Be+ 102 K Na Mg 190 160 Na...
-
The table below lists the ionic radii for the cations and anions in three different ionic compounds. Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of...
-
The table below lists the ionic radii for the cations and anions in three different ionic compounds. Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of...
-
Assume that the average talk time on an Apple iPhone is 20 hours and that this time follows the exponential probability distribution. What is the probability that a randomly selected iPhone will...
-
A farmer made a contract in April to sell to a grain dealer forty thousand bushels of corn to be delivered in October. On June 3, the farmer unequivocally informed the grain dealer that he was not...
-
Alan, an executive with Beta Corporation, follows the principle of rights theory. Under this theory, whether an action is ethical depends on how it affects a. the right determination under a...
-
How do you define what is real? How do you define power? How transparent are your policy-setting processes? How can you contribute to clear assessments of who benefits by decisions made and by what...
-
The Wall Street Journal routinely publishes summaries of corporate quarterly and annual earnings reports in a feature called the Earnings Digest. A typical digest report takes the following form. (a)...
-
A plant asset cost $128.000 and is estimated to have a $16.000 salvage value at the end of its 8-year useful life. The annual depreciation expense recorded for the third year using the...
-
Software Haven sells a software package that is available in three editions. The application should display the price of the edition a customer wants to purchase. The retail prices for the Ultimate,...
-
Potassium chloride has the same crystal structure as NaCl. Careful measurement of the internuclear distance between K + and Cl - ions gave a value of 314.54 pm. The density of KCl is 1.9893 g/cm 3 ....
-
Two views, a top and side view, for the unit cell for rutile (TiO 2 ) are shown here. (a) How many titanium atoms (blue) are in this unit cell? (b) How many oxygen atoms (red) are in this unit cell?
-
According to former Federal Reserve chairman Alan Greenspan, IT investments in the 1990s boosted productivity, which boosted corporate profits, which led to more IT investments, and so on, leading to...
-
1. What is an Interpreted language? 2. What is a dynamically typed language?
-
Q.1 If denotes increasing order of intensity, then the meaning of the words [talk shout scream] is analogous to [please pander]. Which one of the given options is appropriate to fill the blank? (A)...
-
An alkane shows an M+. peak at m/z 114. What is its molecular formula? What will be the relative intensities of the 115/114 peaks?
-
Information graphics, also called infographics, are wildly popular, especially in online environments. Why do you think infographics continue to receive so much attention? How could infographics be...
-
Calculating Annuity Values your company will generate $65,000 in annual revenue each year for the next eight years from a new information database, if the appropriate interest rate is 8.5 percent,...
-
Calculating Annuity Values If you deposit $3,000 at the end of each of the next 20 years into an account paying 10.5 percent interest, how much money will you have in the account in 20 years how much...
-
Calculating Annuity Values you want to have $80,000 in your savings account 10 years from now, and youre prepared to make equal annual deposits into the account at the end of each year, if the...
-
What is the difference between management's goals and the firm's goals? How can the two be in conflict? Provide examples of real world examples of when management and the owners of a company have...
-
Q) A stock price is currently $90. Over each of the next two 6-month periods, it is expected to go up by 10% or down by 10%. The risk-free interest rate is 8% per annum with continuous compounding....
-
A couple who borrow $80,000 for 30 years at 7.2%, compounded monthly, must make monthly payments of $543.03. (Round your answers to the nearest cent.) (a) Find their unpaid balance after 1 year. $...

Study smarter with the SolutionInn App


