The table below lists the ionic radii for the cations and anions in three different ionic compounds.
Question:
The table below lists the ionic radii for the cations and anions in three different ionic compounds.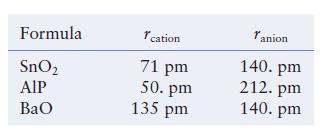 Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of structure formed (NaCl, CsCl, or ZnS) and the type and fraction of holes filled by the cations, and estimate the density of each compound.
Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of structure formed (NaCl, CsCl, or ZnS) and the type and fraction of holes filled by the cations, and estimate the density of each compound.
Transcribed Image Text:
Formula SnO₂ AIP BaO cation 71 pm 50. pm 135 pm Tanion 140. pm 212. pm 140. pm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
the type of structure formed is ZnS Each compound has either the NaCl CsCl or ZnS type cubic structu...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The table below lists the ionic radii for the cations and anions in three different ionic compounds. Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of...
-
The rutile and fluorite structures, shown here (anions are colored green), are two of the most common structure types for ionic compounds where the cation to anion ratio is 1:2. (a) For CaF2 and ZnF2...
-
NaCl and KF have the same crystal structure. The only difference between the two is the distance that separates cations and anions. (a) The lattice energies of NaCl and KF are given in Table 8.2....
-
Suppose there are two identical forest plots except that one will be harvested and left as is while the second will be cleared after the harvest and turned into a housing development. In terms of...
-
The annual per capita consumption of canned vegetables by people in the United States is normally distributed, with a mean of 39 pounds and a standard deviation of 3.2 pounds. Random samples of size...
-
Calculate the price on its issue date of $100,000 face value, 90-day commercial paper issued by GE Capital Canada if the prevailing market rate of return is 0.932%.
-
The figure below is a scatterplot of reading test scores against IQ test scores for 14 fifth-grade children. There is one low outlier in the plot. What effect does this low outlier have on the...
-
Relevance and faithful representation are the qualitative characteristics of useful information under SFAC 8. Evaluate these characteristics from an ethical perspective. That is, how does ethical...
-
bowel mantecures and distributed house points Rainbow Bright for UFO ethod fomation for 2018, 2019 and 2020 is presented in the following mathe icon to view the data) The wave prices for 2018 2017...
-
An analysis of the transactions made by Mark Kotsay & Co., a certified public accounting firm, for the month of August is shown below. The expenses were $650 for rent, $4,800 for salaries and wages,...
-
How does DHsoln relate to deviations from Raoults law? Explain.
-
What type of solid (network, metallic, Group 8A, ionic, or molecular) will each of the following substances form? a. Kr b. SO 2 c. Ni d. SiO 2 e. NH 3 f. Pt
-
With regard to DNA microarrays, answer the following questions: A. What is attached to the slide? Be specific about the number of spots, the lengths of DNA fragments, and the origin of the DNA...
-
Exercise 11-5 Profit allocation in a partnership LO3Dallas and Weiss formed a partnership to manage rental properties, by investing $161,000 and $189,000, respectively. During its first year, the...
-
operation research an introduction by Taha, Hamdy A . 2 0 2 2 . Operations Research - An Introduction. 1 1 th ed . Prentice Hall..kindly explain this
-
which 2 statements are correct regarding budgets in quickbooks online? a . budgets can be created to track capital expenditures. b . budgets can be set up bsed on the last fiscal year's financial...
-
Dr. Yong has requested that Senture Houston, an office manager at Pain Free Dental Associates, prepare a single journal entry for December 31, 2022. The bank statement for that day shows $9,500....
-
This program will not require any IF statements, loops, or custom classes. Instead, it will check inputted data for two kinds of mistakes: Wrong data type (entering text instead of numbers), and math...
-
Fill in the blank(s) to correctly complete each sentence. The graph of (x) = (x - 7) 2 is obtained by shifting the graph of y = x 2 to the______ 7 units.
-
What is the ideal number of children to have? This question was asked on the Sullivan Statistics Survey I. Draw a dot plot of the variable Children from theSullivanStatsSurveyI data set at...
-
Give the expected hybridization of the central atom for the molecules or ions in Exercises 57, 58, and 60 from Chapter 13.
-
Give the expected hybridization of the central atom for the molecules in Exercises 91 and 92 from Chapter 13.
-
Urea, a compound formed in the liver, is one of the ways humans excrete nitrogen. The Lewis structure for urea is Using hybrid orbitals for carbon, nitrogen, and oxygen, determine which orbitals...
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


