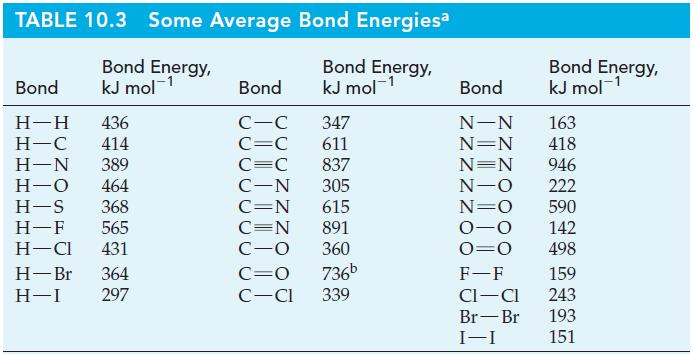
Use data from Table 10.3, but without performing detailed calculations, determine whether each of the following reactions
Question:
Use data from Table 10.3, but without performing detailed calculations, determine whether each of the following reactions is exothermic or endothermic.
![]()
Table 10.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





