Use data from Table 19.1, as necessary, to predict the probable products when Pt electrodes are used
Question:
Use data from Table 19.1, as necessary, to predict the probable products when Pt electrodes are used in the electrolysis of
(a) CuCl2(aq);
(b) Na2SO4(aq);
(c) BaCl2(l);
(d) KOH(aq).
Table 19.1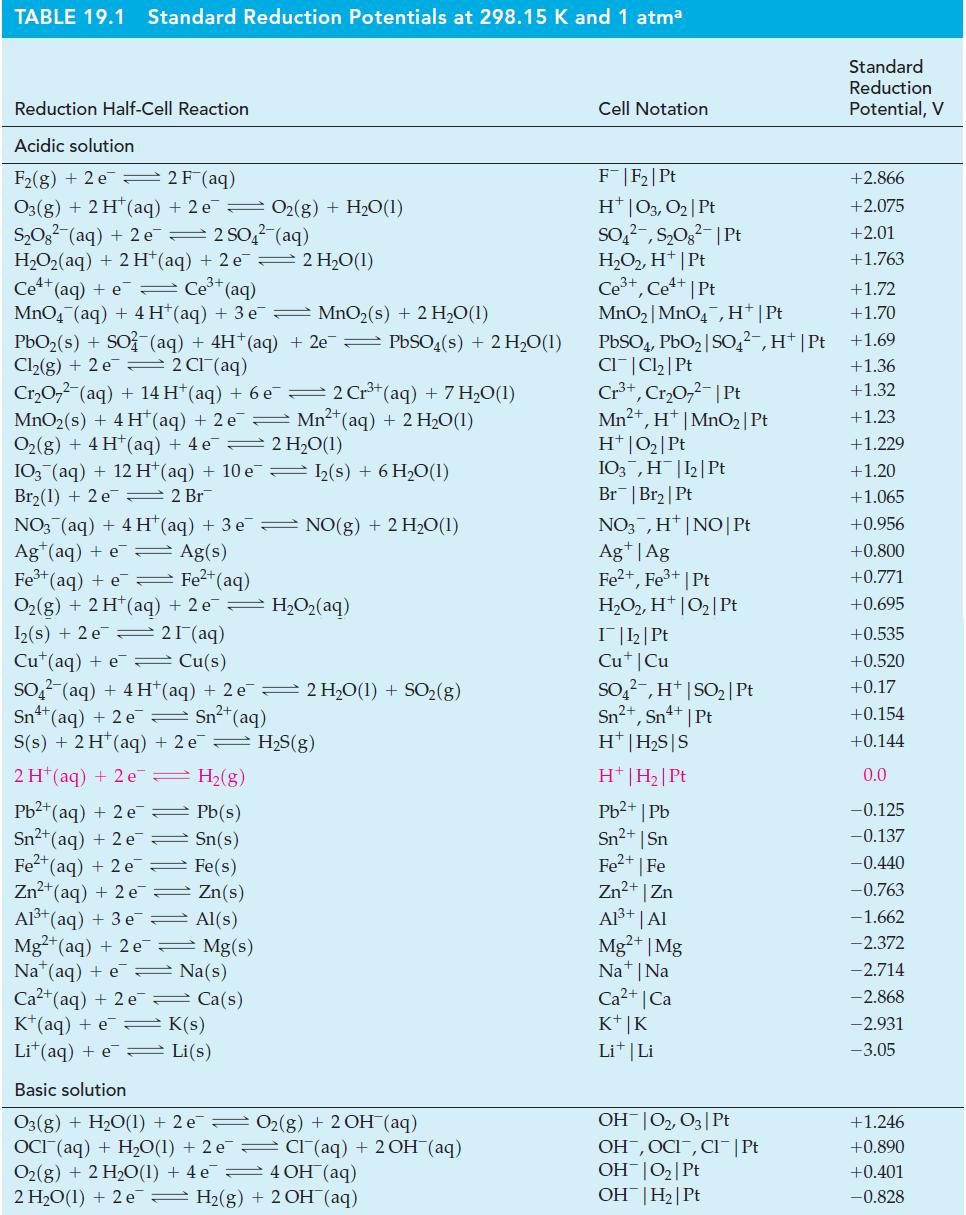
Transcribed Image Text:
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2F (aq)
O3(g) + 2 H¹ (aq) + 2 e 0₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + Ce³+ (aq)
MnO₂ (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂(s) + SO² (aq) + 4H+ (aq) + 2e¯ — PbSO(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 Cl(aq)
Cr₂O7² (aq) + 14 H¹(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4 H* (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4 H(aq) + 4e
2 H₂O(1)
IO3 (aq) + 12 H(aq) + 10 e ₂(s) + 6 H₂0 (1)
Br₂ (1) 2e 2 Br
NO(g) + 2 H₂O(1)
NO3 (aq) + 4H¹ (aq) + 3e¯¯—
Ag (aq) +e
Ag(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2F (aq)
O3(g) + 2 H¹ (aq) + 2 e 0₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + Ce³+ (aq)
MnO₂ (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂(s) + SO² (aq) + 4H+ (aq) + 2e¯ — PbSO(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 Cl(aq)
Cr₂O7² (aq) + 14 H¹(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4 H* (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4 H(aq) + 4e
2 H₂O(1)
IO3 (aq) + 12 H(aq) + 10 e ₂(s) + 6 H₂0 (1)
Br₂ (1) 2e 2 Br
NO(g) + 2 H₂O(1)
NO3 (aq) + 4H¹ (aq) + 3e¯¯—
Ag (aq) +e
Ag(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Electrolysis of CuCl2aq The most likely products of the electrolysis of CuCl2aq are copper metal a...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in the electrolysis of KI(aq). Table 19.1 (B) In the electrolysis of AgNO 3 (aq), what are the expected...
-
Use data from Table to estimate ÎH for the combustion of methane (CH4), as shown below: Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302 121 Si H C O 437 490 9 31222241122...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The accompanying table shows data from the World Bank, World Development Indicators, for real GDP per capita (2010 U.S. dollars) in France, Japan, the United Kingdom, and the United States in 1960...
-
Sealing Company manufactures three types of DVD storage units. Each of the three types requires the use of a special machine that has a total operating capacity of 15,000 hours per year. Information...
-
Table 4-3 contains sample data for parts and for vendors who supply those parts. In discussing these data with users, we find that part numbers (but not descriptions) uniquely identify parts and that...
-
What are the three variations of the simple interest formula?
-
Murray Walter, Inc. (Walter, Inc.) was a general contractor for the construction of a waste treatment plant in New Hampshire. Walter, Inc., contracted with H. Johnson Electric, Inc. (Johnson...
-
the company should buy or produce ? Jenson Monitors Annual Production Costs for 19-inch LCD Monitor Per Unit 10,000 Units Direct Materials $25.00 $250,000 Direct Labor $12.00 $120,000 Variable...
-
An important source of Ag is recovery as a by-product in the metallurgy of lead. The percentage of Ag in lead was determined as follows. A 1.050-g sample was dissolved in nitric acid to produce Pb 2+...
-
The electrodes in the following electrochemical cell are connected to a voltmeter as shown. The half-cell on the right contains a standard silversilver chloride electrode (see Exercise 88). The...
-
Find z and z/w. Leave your answers in polar form. z = cos 80 + i sin 80 w = cos 50 + i sin 50
-
Jason is a sole trader in the architecture industry. For the year ending 30 June 2019, Jason hired a 3D model designer, Sarah, to help him with the growing business. At the end of the year he has the...
-
Read Case 14-1 Trojan Technologies (15th ed., p. 426 OR 16th ed., p. 431) Guiding Questions and additional information: In preparing your case study, ensure that you answer the following questions:...
-
Jorge Rimert works for Road to Success Collection Agency. He oversees mailing out collection notices to patients. Upon review of the patients who have not paid from Hideaway Hospital, Jorge notices...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 99% confident that the estimated percentage is in error...
-
A 100 acre plot of land has a concentration time of 80 minutes. The area is residential single family homes with a C-0.40. What is the percent Increase in stormwater runoff from a 50 year recurrence...
-
Based on the information in Problem 2-13, should you and your roommate specialize in a particular task? Why? And if so, who should specialize in which task? Show how much labor time you save if you...
-
Hardin Services Co. experienced the following events in 2016: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
Fordyce Electronics issues a $400,000, 8%, 10-year mortgage note on December 31, 2009. The proceeds from the note are to be used in financing a new research laboratory. The terms of the note provide...
-
Presented on the next page are three different lease transactions that occurred for Kear Inc. in 2010. Assume that all lease contracts start on January 1, 2010. In no case does Kear receive title to...
-
On July 1, 2010, Atwater Corporation issued $2,000,000 face value, 10%, 10-year bonds at $2,271,813.This price resulted in an effective-interest rate of 8% on the bonds. Atwater uses the...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


