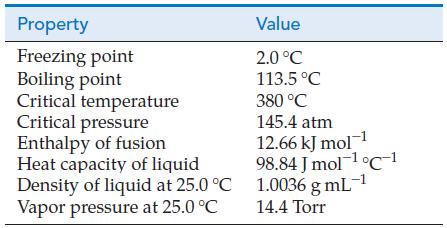
Use data from the table of physical properties of hydrazine, N 2 H 4 , to calculate
Question:
Use data from the table of physical properties of hydrazine, N2H4, to calculate the partial pressure of N2H4(g) when a container filled with an equilibrium mixture of N2H4(g) and N2H4(I) at 25.0 °C is cooled to the temperature of an ice–water bath.
Transcribed Image Text:
Property Freezing point Boiling point Critical temperature Critical pressure Enthalpy of fusion Heat capacity of liquid Density of liquid at 25.0 °C Vapor pressure at 25.0 °C Value 2.0 °C 113.5 °C 380 °C 145.4 atm 12.66 kJ mol-1 98.84 Jmol-¹ °C 1 1.0036 g mL-¹ 14.4 Torr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
Analyze At a temperature below its freezing point of 20 C the hydrazine will be present as a solid i...View the full answer

Answered By

Omar ELmoursi
I'm Omar, I have Bachelor degree in Business and Finance, My unique approach is to help students with questions and assignments, I can teach Business, Math, Accounting, Managerial Accounting, Economy, Human resources management, organizational behavior, project management, I have experience dealing with different types of students and teach them how to deal with different types of exercises.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from the Integrative Example to determine how much heat is required to convert 25.00 mL of liquid hydrazine at 25.0 C to hydrazine vapor at its normal boiling point. Integrative Example Use...
-
A container filled with 45 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer...
-
Below is the comparative income statement of Variline, Inc. Requirements 1. Prepare a horizontal analysis of the comparative income statement of Variline, Inc. Round percentage changes to the nearest...
-
A1 Roofing Co. entered into a written contract with Jaffe to put a new roof on the latters residence for $1,800, using a specified type of roofing, and to complete the job without unreasonable delay....
-
Apply the It formula to the process \(X_{t}:=\sin ^{2}\left(B_{t}ight), t \geqslant 0\).
-
LO2 Why is a tax on real property used more often than a tax on personal property?
-
Missoula Manufacturing Company normally produces 10,000 units of product X each month. Each unit requires 2 hours of direct labor, and factory overhead is applied on a direct labor hour basis. Fixed...
-
An analyst forecasts a firm's nominal Equity Free Cash Flow (EFCF) to be: $10 million next year (t=1); $20 million the year after (t=2); $30 million in 3 years (t=3); and As at year 3, the terminal...
-
Wesley gave his 16-year old daughter, Vivian, several shares of Telus, a taxable Canadian corporation. During the year, Vivian received eligible dividends of $300 from Telus. She sold some of the...
-
What are the types of intermolecular interactions in CH 3 CH 2 NH 2 (l), and which is the strongest?
-
What is the angle between the hybrid orbitals obtained by combining the 2s and two 2p orbitals of an atom? (a) 90 ; (b) 120 ; (c) 180 ; (d) 109.5 ; (e) None of these.
-
State the null hypothesis, Ho, and the alternative hypothesis, Ha, that would be used for a hypothesis test for each of the following statements: a. The mean age of the youths who hang out at the...
-
This week we learned about assessing competition. Watch the video the History of the Cola Wars and answer the following questions. Using the frameworks from the text and the online lesson, why is...
-
Prior to developing your training programs, you must analyze your organizational military needs, identify employee skills gaps based on performance, and have resources available to support training...
-
Describe specifically how your firm's culture lines up with the bullet points listed for that firm . For instance, if you believe your organization's strategy priority is creativity-driven , then...
-
You have recently taken over daycare center that was under substandard leadership. Currently, the staff is unmotivated, negative, and often absent from work. You notice that there is minimal...
-
Choose an organization from the industry of your choice to discuss, illustrate, and reflect deliberately on the following: Why is it important to distinguish between "group" and "team "? What kinds...
-
2-Ethylhexanol, used commercially in the manufacture of plasticizers and synthetic lubricants, is synthesized from butanal via its aldol product. Devise a route to it.
-
Medi-Exam Health Services, Inc. (MEHS), located in a major metropolitan area, provides annual physical screening examinations, including a routine physical, EKG, and blood and urine tests. MEUS's...
-
M&M Propositions how would you answer in the following debate? Q: Isnt it true that the riskiness of a firms equity will rise if the firm increases its use of debt financing? A: Yes, thats the...
-
Optimal Capital Structure is there an easily identifiable debt-equity ratio that will maximize the value of a firm? Why or why not?
-
Financial Leverage why is the use of debt financing referred to as financial leverage?
-
The price of a vacation home is currently $314,041. If the price of vacation homes is increasing at a rate of 3.45% per year, how much would a vacation home cost in 9 years?
-
1a) AA Corporation's stock has a beta of 0.8. The risk-free rate is 4%, and the expected return on the market is 12%. What is the required rate of return on AA's stock? Do not round intermediate...
-
Your are required to present one or two audit issue (s) based on the topic of *Important issues in financial statement audit*. 1. The primary goal of your audit issue(s) is not to judge whether the...

Study smarter with the SolutionInn App


