Use data from the Integrative Example to determine how much heat is required to convert 25.00 mL
Question:
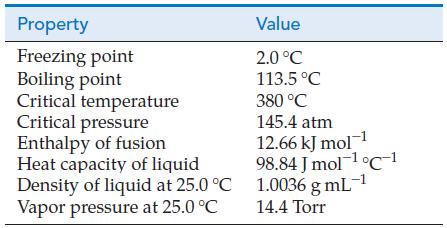
Use data from the Integrative Example to determine how much heat is required to convert 25.00 mL of liquid hydrazine at 25.0 °C to hydrazine vapor at its normal boiling point.
Integrative Example
Use data from the table of physical properties of hydrazine, N2H4, to calculate the partial pressure of N2H4(g) when a container filled with an equilibrium mixture of N2H4(g) and N2H4(I) at 25.0 °C is cooled to the temperature of an ice–water bath.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





