Question: Use data from the text to construct a standard electrode potential diagram relating the following vanadium species in acidic solution. ta vo VO2+ V3+ V2+
Use data from the text to construct a standard electrode potential diagram relating the following vanadium species in acidic solution.
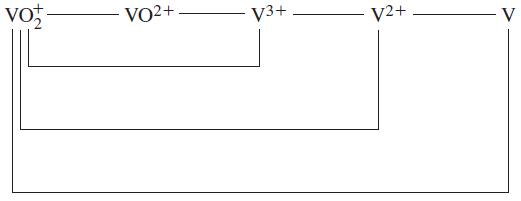
ta vo VO2+ V3+ V2+ V
Step by Step Solution
3.30 Rating (165 Votes )
There are 3 Steps involved in it
Based on the information provided in the image the following standard electrode potential diagram ca... View full answer

Get step-by-step solutions from verified subject matter experts


