Question: Use data from the text to construct a standard electrode potential diagram relating the following chromium species in acidic solution. Cr0- Cr+ Cr2+ - Cr
Use data from the text to construct a standard electrode potential diagram relating the following chromium species in acidic solution.
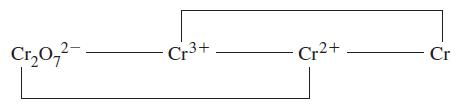
Cr0- Cr+ Cr2+ - Cr
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Based on the information provided in the text and the image the following standard electrode potenti... View full answer

Get step-by-step solutions from verified subject matter experts


