Use Hesss law and the following data CH4(g) + 2O2(g) CH4(g) + CO(g) CH(g) +
Question:
Use Hess’s law and the following data
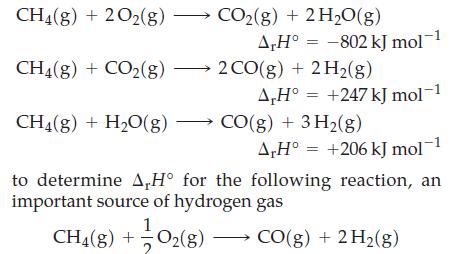
Transcribed Image Text:
CH4(g) + 2O2(g) → CH4(g) + CO₂(g) — CH₂(g) + H₂O(g) CO₂(g) + 2H₂O(g) A,H° -802 kJ mol 2 CO(g) + 2H₂(g) A,H° = +247 kJ mol-1 CO(g) + 3H₂(g) A,H° +206 kJ mol−1 to determine A,H° for the following reaction, an important source of hydrogen gas CH4(g) + O₂(g) — CO(g) + 2 H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
To use Hesss law to determine the enthalpy change for the reaction CH4g 12O2g COg 2H2g We can use th...View the full answer

Answered By

Rajat Gupta
used to take tution classes from my school time.
Conducted special topic claases during my graduation to help the students pass their exams.
Currently, teaching and conducting online claases during my post- graduation too.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following is the monthly payroll of Johnson Company, owned by Dan Johnson. Employees are paid on the last day of each month. Johnson Company is located at 2 Square Street, Marblehead,...
-
John Andrews, the accountant for Johnson Company, must complete Form 941 for the first quarter of the current year. John gathered the needed data as presented in Problem 8A-2. Suddenly called away to...
-
Gebruik Hess se Wet om die reaksiewarmte vir die proses te bepaal. Use Hess's Law to determine the heat of reaction for the process. CH4(g) + 6HCl(g) 2CHCl3(g) + 4H(g) i. 2C(s) + 2H(g) CH4(g) | AH =...
-
Which of the following is most nearly the mass of the Earth? (The radius of the Earth is about 6.4 106 m) A. 6 x 1024 kg B. 6 x 107 kg C. 6 x 103 kg D. 6 x 1033 kg E. 6 x 1036 kg
-
Many fast-food restaurants compete on lean business concepts. Match each of the following activities at a fast-food restaurant with the lean business concept it strives to achieve. Some activities...
-
How might the existence of factor-intensity reversals be a reason for the Leontief paradox? How might the existence of demand reversal?
-
PAYBACK PERIOD Refer to Problem 11-1. What is the projects payback? AppendixlLO1
-
Dusty Johnson is the accounting and finance manager for a manufacturer. At year- end, he must determine how to account for the companys contingencies. His manager, Tom Pretti, objects to Johnsons...
-
Question 2 (15 marks) David Wong, the product manager of KiKi Company, was reviewing the production schedule for the last quarter of 2020. He noted that the company planned to sell 4,000 units during...
-
Ricardo works for Bank B and is talking about loans with a consumer. He provides the consumer with a general explanation regarding the basic qualifications of a loan. Although the consumer plans to...
-
The standard heats of combustion ( r H) of buta-1,3-diene, C 4 H 6 (g); butane, C 4 H 10 (g); and H 2 (g) are -2540.2, -2877.6, and -285.8 kJ mol-1, respectively. Use these data to calculate the heat...
-
CCl 4 , an important commercial solvent, is prepared by the reaction of Cl 2 (g) with a carbon compound. Determine r H for the reaction CS(1) + 3Cl(g) CCl4(1) + SCl2 (1) Use appropriate data from...
-
Do you think sometimes managers are justified in not taking their employees advice? Why or why not? LO8-2
-
Solve: (5)*+1 = 25x
-
The ball bearing made of steel is to be heat treated. It is heated to a temperature of 620C and then quenched in water that is at a temperature of 50C. The ball bearing has a diameter of 5 cm and the...
-
1. Using the net present value? method, calculate the comparative cost of each of the three payment plans being considered by New Med 2. Which payment plan should New Med choose? Explain. 3. Discuss...
-
Swenson Company produced 300 units in year one and sold 260 units in that year. In year two, it produced 260 units and sold 300 units. Total fixed overhead was the same in years one and two. Under...
-
c) Determine the maximum rotational speed such that the fluid will not spill over the container. (and: = 2gh/R) [2 marks] d) The container in Figure 4 now contains coffee (p~1000) which is 7cm deep...
-
Why is CBN better for machining steel than diamond?
-
If the jobs displayed in Table 18.24 are processed using the earliestdue-date rule, what would be the lateness of job C? TABLE 18.24 Processing Times and Due Dates for Five Jobs Job C D E...
-
An entry to record Purchases and related Accounts Payable of $13,000 for merchandise purchased on December 23, 2011, was recorded in January 2012. This merchandise was not included in inventory at...
-
Equipment was purchased on January 2, 2010, for $24,000, but no portion of the cost has been charged to depreciation . The corporation wishes to use the straight-line method for these assets, which...
-
Where can authoritative iGAAP related to accounting changes be found?
-
What does it mean by standard hours per unit and standard rate per unit in variable manufacturing overhead standards.
-
On March 1, 2019, Cool Inc. paid $960,000 to acquire a 35 percent investment in Mint Ltd. After one year, Mint Ltd. reported net income of $260,000 for the first year and declared and paid cash...
-
equired: 1. Select a company registered in the Stock Exchange Istanbul (BIST) for manufacturing companies. 2. After specifying your company, specify the basic characteristics of the company as...

Study smarter with the SolutionInn App


